1. Blaser MJ. Helicobacter pylori and gastric diseases. BMJ. 1998; 316:1507–1510.
2. Rhee KH, Youn HS, Baik SC, Lee WK, Cho MJ, Choi HJ, Maeng KY, Ko KW. Prevalence of Helicobacter pylori infection in Korea. J Korean Soc Microbiol. 1990; 25:475–490.
3. Gologan A, Graham DY, Sepulveda AR. Molecular markers in Helicobacter pylori-associated gastric carcinogenesis. Clin Lab Med. 2005; 25:197–222.
4. Seo JH, Park JS, Yeom JS, Lim JY, Park CH, Woo HO, Baik SC, Lee WK, Cho MJ, Rhee KH, et al. Correlation between positive rate and number of biopsy samples on urease test in childhood Helicobacter pylori infection. J Korean Med Sci. 2014; 29:106–109.
5. Crabtree JE, Mahony MJ, Taylor JD, Heatley RV, Littlewood JM, Tompkins DS. Immune responses to Helicobacter pylori in children with recurrent abdominal pain. J Clin Pathol. 1991; 44:768–771.
6. Kindermann A, Konstantopoulos N, Lehn N, Demmelmair H, Koletzko S. Evaluation of two commercial enzyme immunoassays, testing immunoglobulin G (IgG) and IgA responses, for diagnosis of Helicobacter pylori infection in children. J Clin Microbiol. 2001; 39:3591–3596.
7. Oleastro M, Matos R, Cabral J, Barros R, Lopes AI, Ramalho P, Monteiro L. Evaluation of a Western blot test, Helico Blot 2.1, in the diagnosis of Helicobacter pylori infection in a pediatric population. Helicobacter. 2002; 7:210–215.
8. Akada J, Okuda M, Hiramoto N, Kitagawa T, Zhang X, Kamei S, Ito A, Nakamura M, Uchida T, Hiwatani T, et al. Proteomic characterization of Helicobacter pylori CagA antigen recognized by child serum antibodies and its epitope mapping by peptide array. PLoS One. 2014; 9:e104611.
9. Seo JH, Lim CW, Park JS, Yeom JS, Lim JY, Jun JS, Woo HO, Youn HS, Baik SC, Lee WK, et al. Correlations between the CagA antigen and serum levels of anti-Helicobacter pylori IgG and IgA in children. J Korean Med Sci. 2016; 31:417–422.
10. Haas G, Karaali G, Ebermayer K, Metzger WG, Lamer S, Zimny-Arndt U, Diescher S, Goebel UB, Vogt K, Roznowski AB, et al. Immunoproteomics of Helicobacter pylori infection and relation to gastric disease. Proteomics. 2002; 2:313–324.
11. Jung HS, Kim EJ, Kim EA, Park JH, Jun JS, Seo JH, Lim JY, Choi MB, Woo HO, Youn HS, et al. Detection of Helicobacter pylori by pre-embedding immunoelectron microscopy: comparison with immunoblotting method. J Korean Pediatr Soc. 2002; 45:862–874.
12. Cho MJ, Jeon BS, Park JW, Jung TS, Song JY, Lee WK, Choi YJ, Choi SH, Park SG, Park JU, et al. Identifying the major proteome components of Helicobacter pylori strain 26695. Electrophoresis. 2002; 23:1161–1173.
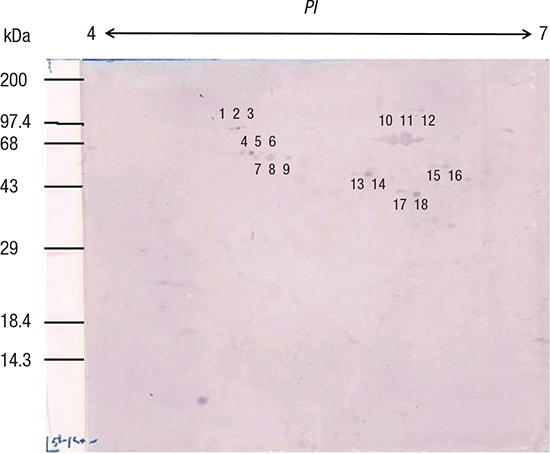
13. Jung TS, Kang SC, Choi YJ, Jeon BS, Park JW, Jung SA, Song JY, Choi SH, Park SG, Choe MY, et al. Two-dimensional gel electrophoresis of Helicobacter pylori for proteomic analysis. J Korean Soc Microbiol. 2000; 35:97–108.
14. O’Connell KL, Stults JT. Identification of mouse liver proteins on two-dimensional electrophoresis gels by matrix-assisted laser desorption/ionization mass spectrometry of in situ enzymatic digests. Electrophoresis. 1997; 18:349–359.
15. Lin YF, Chen CY, Tsai MH, Wu MS, Wang YC, Chuang EY, Lin JT, Yang PC, Chow LP. Duodenal ulcer-related antigens from Helicobacter pylori: immunoproteome and protein microarray approaches. Mol Cell Proteomics. 2007; 6:1018–1026.
16. Lin YF, Wu MS, Chang CC, Lin SW, Lin JT, Sun YJ, Chen DS, Chow LP. Comparative immunoproteomics of identification and characterization of virulence factors from Helicobacter pylori related to gastric cancer. Mol Cell Proteomics. 2006; 5:1484–1496.
17. Mitchell HM, Hazell SL, Kolesnikow T, Mitchell J, Frommer D. Antigen recognition during progression from acute to chronic infection with a cagA-positive strain of Helicobacter pylori
. Infect Immun. 1996; 64:1166–1172.
18. Kim EA, Kim YO, Lim JY, Jung YS, Park CH, Woo HO, Youn HS, Ko GH, Baik SC, Lee WK, et al. Antibody response of infants to Helicobacter pylori infection. Korean J Gastroenterol. 2000; 35:704–715.
19. Lock RA, Coombs GW, McWilliams TM, Pearman JW, Grubb WB, Melrose GJ, Forbes GM. Proteome analysis of highly immunoreactive proteins of Helicobacter pylori
. Helicobacter. 2002; 7:175–182.
20. Suerbaum S, Josenhans C, Labigne A. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol. 1993; 175:3278–3288.
21. Labigne A, Cussac V, Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991; 173:1920–1931.
22. Narikawa S, Imai N, Yamamoto M, Suzuki T, Yanagawa A, Mizushima Y. Oxygen and carbon dioxide requirements of Helicobacter pylori
. Acta Microbiol Immunol Hung. 1995; 42:367–371.
23. Schmitt L, Tampé R. Structure and mechanism of ABC transporters. Curr Opin Struct Biol. 2002; 12:754–760.
24. Wang D, Luo B, Shan W, Hao M, Sun X, Ge R. The effects of EF-Ts and bismuth on EF-Tu in Helicobacter pylori: implications for an elegant timing for the introduction of EF-Ts in the elongation and EF-Tu as a potential drug target. Metallomics. 2013; 5:888–895.
25. Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999; 397:176–180.








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download