INTRODUCTION
Tuberculosis (TB) is one of the major public health problems in Democratic People's Republic of Korea (North Korea). The estimated incidence of TB is 442/100,000 population (
1). Although no nationwide epidemiological data have been obtained yet, drug-resistant TB can be the most important hurdle in TB control in North Korea. Given very limited second-line drugs (SLDs) supplies, only a small portion of patients can be treated with SLD through the Global Drug Facility (GDF) under the Programmatic Management of Drug-resistant TB (PMDT) which started in 2012 (
2) or the Eugene Bell Foundation (EBF) which commenced in 2008 (
3). Therefore, most patients, even those who failed with the category II drug treatment and those suspected for multidrug-resistant (MDR)-TB, would be repeatedly treated with first-line drugs especially before 2008. Therefore, the resistance distribution can be different in other regions of the world or from our expectation.
The treatment success of new sputum smear-positive patients has consistently exceeded 80% since 2001 and 90% since 2009, after the Ministry of Public Health (MOPH) adopted directly observed therapy short course (DOTS) in North Korea in 1998 (
2) with the help of international aids including the Global Fund to Fight AIDS, TB and Malaria (GFATM) and United Nations Children's Fund (UNICEF) (
2). However, few of these international aids have included treatment for drug-resistant TB. A national or subnational drug resistance survey has not been performed in North Korea yet. However, limited drug resistance surveillance survey was conducted recently in North Hwanghae Province. The interim results of this limited survey showed that rifampicin (RIF) resistance was detected in 2.2% and 16.3% of new and previously treated smear-positive patients, respectively (
2). Therefore, we can assume that significant number of MDR-TB patients exist in North Korea.
The EBF requested the Clinical Research Center of the Masan National Tuberculosis Hospital (MNTH) for culture and drug susceptibility test (DST) of sputum specimens obtained from TB patients in the sanatoria of North Korea from 2007 to 2009. The EBF is a United States and Republic of Korea (South Korea) based non-governmental organization that supports for diagnosis and treatment of MDR-TB and drug-susceptible TB patients in the sanatoria that spans North Korea (
4). EBF has been visiting each center once every 6 months to deliver sputum samples for DST since 2007 (
5), and supplying SLD to North Korea since 2008.
This study aims to analyze the drug resistance patterns of Mycobacterium tuberculosis isolated from the sputum samples of the patients who were recorded to be untreated with SLD in the sanatoria of North Korea, during the period when SLD was not officially supplied to North Korea.
MATERIALS AND METHODS
Collection and transfer of sputum specimens
The EBF staff visited North Korean sanatoria and delivered sputum specimens of TB patients to the MNTH every 6 months from 2007 to 2009. The sanatoria (n = 17) were located in the capital city area and rural areas close to the border to China and South Korea. Before each EBF visit, potential candidates for MDR-TB are selected by the sanatorium staff from those who have failed to respond to the standard DOTS regimens (category I or II). The EBF staff registered the patients by taking photos with name, sex, and age of each patient for identification purposes and recorded the clinical information of the patients. However, not all patients provided these data.
Because the period from sputum collection to culture took approximately over 2 weeks, the following protocol was applied to improve bacterial viability. Sputum specimens were collected in 50 mL conical tubes and mixed with equal volume of 1% (w/v) cetylpyridinium chloride (Sigma-Aldrich, St. Louis, MO, USA) for decontamination and were shaken by hand (
6-
8). Subsequently, the sputum specimens were kept in room temperature and transferred to the MNTH when the EBF staff returned to South Korea. The average time interval between sputum sampling and transfer to the MNTH was 11.4 (range of 5–20) days.
Pretreatment and acid-fast bacillus stain/culture
After transfer to the MNTH, the sputum specimens were neutralized with the same volume of neutralizing buffer (Difco™; Becton, Dickinson and Company, Detroit, MI, USA) as recommended by the manufacturer and centrifuged at 5,000 × g for 10 minutes. After discarding the supernatant, the pellet was resuspended with 1 mL phosphate-buffered saline (pH 6.8). Exactly 100 µL of the resuspension was plated on 3% Ogawa slant culture medium. The plated culture media were placed in a 37°C incubator and observed daily. Culture negative was defined if the absence of organism growth after 8 weeks (
9).
Cultured organisms were confirmed as M. tuberculosis via TB antigen testing using SD BIOLINE TB Ag MPT64 Rapid® Kit (SD BIOLINE; Standard Diagnostics, Inc., Yongin, Korea). Negative result on TB Ag MPT64 and growth of bacterial colonies on the paranitrobenzoic acid containing Löwenstein-Jensen (L-J) medium was considered as nontuberculous mycobacteria.
DST in L-J medium
Culture-based conventional phenotypic DST was performed using the absolute concentration method in L-J medium, which was commercially provided by the Korean Institute of Tuberculosis (Osong, Korea) based on the manufacturer's instructions. The critical drug concentrations were set by following the World Health Organization's (WHO) guideline; 0.2 µ/mL isoniazid (INH), 40.0 µ/mL RIF, 2.0 µ/mL ethambutol (EMB), 10.0 µ/mL streptomycin (S), 40.0 µ/mL injectable drugs (IDs) (kanamycin [Km], amikacin [Am], and capreomycin [Cm]), 2.0 µ/mL fluoroquinolones (FQs) (ofloxacin [Ofx] and moxifloxacin [Mfx]), 1.0 µ/mL para-aminosalicylic acid (PAS), 40.0 µ/mL ethionamide (Eto), and 30.0 µ/mL cycloserine (Cs). Bacterial growth was checked every week. The growth of M. tuberculosis was confirmed using TB Ag MPT64 Rapid® Kit (Standard Diagnostics, Inc.). M. tuberculosis was confirmed to be resistant when it grew in the drug-containing medium and as sensitive in the absence of bacterial growth for 8 weeks. The resistance of M. tuberculosis strains to pyrazinamide (PZA) was tested using pyrazinamidase assay.
We analyzed the DST results of M. tuberculosis strains isolated from the patients' sputum specimens that were transferred only for the first time to exclude the effects of SLD supplied by the EBF. The EBF started to treat the patients with the regimen prescribed based on the DST data that included SLD. To eliminate the presence of any possible effects of the treatment on the drug resistance patterns of TB, M. tuberculosis strains isolated for the second time or more from the same patients were excluded in this study. Therefore, the DST results in this study were for M. tuberculosis strains that were isolated from the patients who may have not been treated with SLD before.
Statistical analysis
The statistical significance was analyzed through χ2 test using GraphPad InStat software (version 3.0; GraphPad Software, Inc., La Jolla, CA, USA). P values < 0.05 were considered as significantly different.
Ethics statement
The EBF staff collected sputum samples from patients in the sanatoria and transferred these samples to the MNTH for isolation and DST of M. tuberculosis strains from 2007 to 2009 for treatment purposes. Considering that the culture and DST results of the M. tuberculosis strains isolated from the sputum samples were retrospectively analyzed, this research was approved by the Institutional Review Board of the MNTH (IRB-12-N02) with the exemption of informed consent.
DISCUSSION
Most patients with pulmonary TB in the sanatoria of North Korea who failed category I or II drug treatment were MDR-TB patients. Over 3 quarters of the suspected cases were, in fact, MDR-TB. M. tuberculosis strains which were obtained from the patients in the sanatoria had high resistance rates for INH, RIF, EMB, and S, but only low resistance rate for FQs. Furthermore, significant regional differences were observed in the resistance rates for RIF, FQs, and IDs. Big city regions had higher resistance rates for RIF, FQs, and IDs except for S than the rural areas.
In this study, the INH resistance rate was over 90%, and the RIF and S resistance rates were over 70%. The fact that these were the only drugs supplied to North Korea may be one of the reasons for these high resistance rates to first-line drugs (
10). Currently, the Global Fund allocates major budget for TB control in North Korea. GDF, a WHO-led initiative that has supplied high-quality drugs to over 90 countries provide treatment kits for adults in North Korea. The GDF standard treatment kit for new patients (category I) consists of 2 months of INH, RIF, EMB, and PZA, followed by 4 months of INH and RIF. The retreatment kit (category II) consists of 2 months of S injections plus INH, RIF, EMB, and PZA, followed by 1 month of INH, RIF, EMB, and PZA and 5 months of INH, RIF, and EMB. Therefore, the patients had to be repeatedly treated with first-line drugs even after failure of treatment. North Korea reported 85,564 new TB patients in 2011, the vast majority of whom would have received the GDF standard treatment kit for new patients (category I). Additionally, 13,507 patients with a history of TB treatment would have also received the GDF standard retreatment kit (category II) (
4). Therefore, a significant portion of patients who received category II drugs can be assumed to already be suffering from MDR-TB; hence, the drugs were less effective. The EBF started to supply SLD since 2008 and PMDT has also been supplying SLD since June 2012 (
2). This study was performed in the time period between 2007 and 2009 during which SLD were hardly supplied. Even after the EBF supplied SLD, the sputum specimens were collected at the sanatoria where SLD were not provided yet.
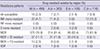
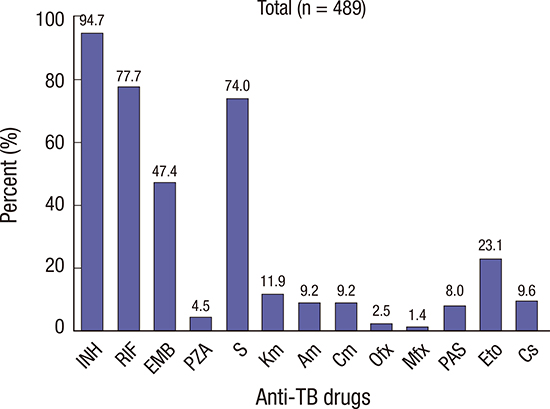
In this study (n = 489), the resistance to INH, RIF, EMB, and S was found in 463 (94.7%), 380 (77.7%), 232 (47.4%), and 362 (74.0%) patients, respectively. On the contrary, the SLD resistance rate was very low. We could not directly compare the resistance rates between North and South Korea, because most of South Korean data included all patients with TB and the data of this study represented the patients who failed the first-line drug treatment. However, North Korea showed a considerably larger difference in resistance rate between first-line drugs and SLD than South Korea (
11).
These high resistance rates to first-line drugs were similar to those in the report of Seung and Linton in 2013 (
4). However, they reported that the Ofx resistance rate was 11% which is significantly higher than the result of this study (2.5%). The difference can be attributed to the fact that SLD was supplied since 2008. They evaluated the resistance pattern from April 2010 to April 2011. However, the period of this study is from 2007 to 2009 and sputum specimens were collected from patients who were recorded to be untreated with SLD. That is, in this study, we evaluated the DST results of
M. tuberculosis strains isolated from sputum specimens that were only collected for the first time to exclude the effects of SLD supplied by the EBF. Therefore, the resistance pattern would become more variable when the supply of SLD increases and rapid and exact DST for the first-line drugs and SLD would be needed.
The drug resistance pattern of TB shown in this study cannot represent the actual state of the pattern in North Korea. However, this drug resistance pattern suggests that MDR-TB in the sanatoria would be very serious and the TB patients who have failed the standard DOTS regimen would take SLD by informal systems (
4). Especially, the patients in the capital city area (region A) and close to the border of China (region B) can be speculated to have easier access to SLD than those in relatively isolated area (region C) when considering the resistance rates of SLD and detection of XDR-TB patients (
Table 1,
Fig. 1).
In North Korea, one of the major problems in TB control is unavailability of consistently proficient culture and DST laboratories (
2). These are necessary to treat drug-resistant TB with effective individualized regimen, especially when drug resistance patterns can be more variable in the future.
Although PZA is a member of category I and II drugs, resistance to PZA was very low (22 isolates, 4.5%). The reason for this low resistance is not definite although, the possible cause could be the short period of exposure or laboratory methods. The high resistance rate to S and the low resistance rate to other IDs and FQs were associated with S being provided as category II drug and other IDs and FQs not being officially supplied to North Korea during the period of this study. Although, FQ resistance rate was generally very low, regional variations were observed. The resistance to these drugs seemed to be higher in large city than those in other relatively rural areas. Region C (southern part of North Korea) had lower MDR-TB rate than the other regions. The exact reason has not been well evaluated. However, we can postulate that one of the reasons is that patients who live in big cities can have more chances of exposure to these drugs.
A few limitations exist in this study. First, the results of this study cannot be generalized to estimate the exact drug resistance pattern of all TB patients in North Korea or even in the sanatoria, because MDR-TB patients were selected and registered for sputum collection from those who have failed to respond to the standard DOTS regimens (category I or II). Moreover, clear and specific criteria for patient selection were not developed. Second, little clinical information of the patients was available. Additionally, no data was available even on the previous regimens, and number of previous treatments. However, the resistance pattern found in this study is still valuable information because only little data on drug-resistant TB in North Korea exist.
In conclusion, small but significant regional variations in resistance pattern of M. tuberculosis from the sanitaria in North Korea were observed. The INH and RIF resistance rates were especially high at 94.7% and 77.7%, respectively. On the other hand, the resistance rates for FQ and IDs except for S were very low. However, this study was performed only for selected patients in the sanatoria in North Korea. Therefore, further studies are needed to evaluate the exact resistance pattern of TB in North Korea.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download