INTRODUCTION
MATERIALS AND METHODS
Establishment of the synonym database
Supplementation of K-LOINC's clinical microbiology section
K-LOINC's compatibility with other laboratory code systems
RESULTS
Table 1
Final products of the standardization for laboratory terminology

Establishment of the synonym database
Supplementation of K-LOINC's clinical microbiology section
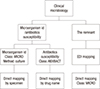
After filtering clinical microbiology codes, classify them into microorganism identification, antibiotics susceptibility tests, and the remainder.
In general, the remaining codes can be mapped using EDI codes.
When a remaining code cannot be mapped to an EDI code, map it directly after selecting the K-LOINC class as “MICRO.”
For microorganism identification, attempt to map directly using the specimen name, by selecting the appropriate “system” (e.g., sputum) and “method” (usually “culture”) after filtering the K-LOINC class as “MICRO.”
For an antibiotics susceptibility test, attempt to map directly using the drug name, by selecting the appropriate “method” (e.g., MIC) after filtering the K-LOINC class as “ABXBACT.”




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download