INTRODUCTION
CASE DESCRIPTION
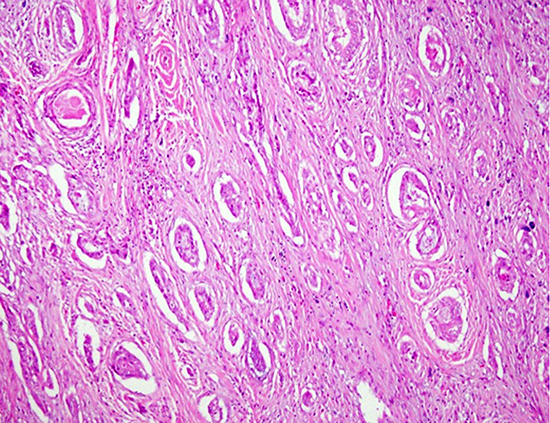
Case 1
Fig. 1
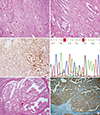
Table 1
Immunohistochemical staining and BRAF mutation results

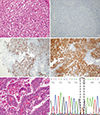
Case 2
Fig. 2
Journal List > J Korean Med Sci > v.32(10) > 1108400



Young Shin Song 
https://orcid.org/0000-0003-4603-1999
Chan Kwon Jung 
https://orcid.org/0000-0001-6843-3708
Kyeong Cheon Jung 
https://orcid.org/0000-0002-7741-7184
Young Joo Park 
https://orcid.org/0000-0002-3671-6364
Jae-Kyung Won 
https://orcid.org/0000-0003-1459-8093