This article has been corrected. See "Erratum: Environmental Heavy Metal Exposure and Chronic Kidney Disease in the General Population" in Volume 30 on page 507.
Abstract
Lead (Pb), mercury (Hg), and cadmium (Cd) are common heavy metal toxins and cause toxicological renal effects at high levels, but the relevance of low-level environmental exposures in the general population is controversial. A total of 1,797 adults who participated in the KNHANES (a cross-sectional nationally representative survey in Korea) were examined, and 128 of them (7.1%) had chronic kidney disease (CKD). Our study assessed the association between Pb, Hg, Cd exposure, and CKD. Blood Pb and Cd levels were correlated with CKD in univariate logistic regression model. However, these environmental heavy metals were not associated with CKD after adjustment for age, sex, BMI, smoking, hyperlipidemia, hypertension, diabetes, and these metals in multivariate logistic regression models. We stratified the analysis according to hypertension or diabetes. In the adults with hypertension or diabetes, CKD had a significant association with elevated blood Cd after adjustment, but no association was present with blood Pb and Hg. The corresponding odds ratio [OR] of Cd for CKD were 1.52 (95% confidence interval [CI], 1.05-2.19, P=0.026) in adults with hypertension and 1.92 (95% CI, 1.14-3.25, P=0.014) in adults with diabetes. Environmental low level of Pb, Hg, Cd exposure in the general population was not associated with CKD. However, Cd exposure was associated with CKD, especially in adults with hypertension or diabetes. This finding suggests that environmental low Cd exposure may be a contributor to the risk of CKD in adults with hypertension or diabetes.
Chronic kidney disease (CKD) is a global public health problem because of a significant increase in prevalence, the enormous cost of management, and its role as a risk factor for cardiovascular disease. Diabetes and hypertension are the major risk factors of CKD. Many other risk factors of cardiovascular disease, such as age, obesity, smoking, and hypercholesterolemia are linked to CKD (1). Environmental heavy metals such as lead (Pb), mercury (Hg), and cadmium (Cd) are established nephrotoxins at high exposure levels (2, 3, 4, 5, 6, 7). Chronic exposure to low levels is widespread in the current environment, especially in industrializing countries, and these metals slowly accumulate in the body. In particular, environmental Pb, Hg, and Cd exposure can occur widespread in air, food, cigarettes, gasoline, contaminated crops, and seafood (3, 6). However, their relevance at environmental low-level exposures in the general population is controversial.
Substantial experimental evidence suggests that exposure to Cd may cause oxidative stress, inflammation, and lipid peroxidation in the organs (5, 8). Chronic low exposure to Cd can cause both renal proximal tubular damage and decline in glomerular filtration rate (GFR) in experimental animal models (9). Human and animal studies reported that Cd may potentiate or exacerbate diabetic nephropathy (10). Pb toxicity has been shown to cause mitochondrial swelling in the renal tubular cells and impair energy production (7). Information about the relationship between low levels of environmental Hg exposure and decreased renal function is scarce, but occupational exposure to elemental Hg vapor has been shown to cause albuminuria and severe membranous nephropathy (11).
Therefore, we evaluated the association of blood Pb, Hg, and Cd level with CKD in Korean adults who participated in the fifth Korean National Health and Nutrition Examination Survey (KNHANES V-2, 2011), Korea Centers for Disease Control and Prevention. To evaluate the effect of other CKD risk factors on the renal toxicity of these metals, we stratified and examined the association among participants according to hypertension or diabetes.
The KNHANES is a cross-sectional nationally representative survey of the general population in Korea, and is regularly conducted by the Korea Center of Disease Control and Prevention (KCDC) in the Ministry of Health and Welfare. KNHANES was performed year-round, which is a similar data collection method to that used in the US NHANES. KNHANES data are publicly available (website: https://knhanes.cdc.go.kr/knhanes/index.do). All participants provided written informed consent. The fifth Korea National Health and Nutrition Examination Survey (KNHANES V-2, 2011) was conducted from January 2011 through December 2011. The KNHANES V-2 consisted of health questions and a health screening via a standardized questionnaire followed by a detailed physical examination and laboratory test at a mobile examination center and nutrition questions via standardized questionnaires administered in the home by trained interviewers. The participants were chosen from the candidates by using proportional allocation-systemetic sampling with multistage stratification (age, sex and region) (n=8,518). Blood Pb, Hg and Cd were measured in some participants by probability sampling (n=2,395). We included participiants ≥20 yr of age in the analysis. We excluded pregnant women and those missing serum creatinine, and urine albumin data and other variables of interest, finally leaving 1,797 adults with complete data.
Blood Pb and Cd levels were determined by graphite furnace atomic absorption spetrophotometry (GFAAS) using AAnalyst 600 (PerkinElmer, Turku, Finland) and blood Hg levels, gold-amalgamation process using DMA-80 (Milestone, Milano, Italy).
Data relevant to the analysis included age, sex, body mass index (BMI), self-reported information about cigarette smoking, and whether subjects were diagnosed with hypertension or diabetes by a physician. Serum and urine samples were collected at a mobile examination center; and HbA1c, fasting glucose, creatinine, blood Pb, Hg, and Cd were measured.
Three blood pressure measurements were taken at the mobile examination center by a trained and certified observer following the KNHANES protocol. The average of the second and third blood pressure measurements were used for the data analysis. Hyperlipidemia was defined as a diagnosis history of hyperlipidemia or antihyperlipidemic medication. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or diagnosis history of hypertension. Diabetes mellitus was defined as serum HbA1c ≥6.5%, fasting glucose ≥126 mg/dL, or diagnosis history of diabetes.
CKD was defined as estimate glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or urine albumin-to-creatinine ratio (ACR) ≥30 mg/g, the standard definition of CKD, as defined in the most recent Kidney Disease Improving Global Outcome (KDIGO) 2012 guidelines. A morning urine sample was obtained from a random subsample of participants for urine albumin and creatinine. Urinary albumin levels were measured by turbidimetric assay using a Hitachi Automatic Analyzer 7600 (Hitachi, Koka-city, Japan) and serum creatinine levels were measured by a enzymatic colorimetric method. eGFR was calculated from the Modification of Diet In Renal Disease (MDRD) formula, adjusting serum creatinine for age and sex. eGFR (mL/min/1.73 m2)=175×SCr-1.154×age-0.203×0.742 (if female)×1.21.
Data are expressed as mean±standard deviation or frequencies (%). Baseline characteristics were compared with chi-square test or Fisher's exact test for categorical variables and Student's t-test for continuous variables. We constructed multivariate logistic regression models to identify independent factors associated with CKD. Univariate and multivariate analysis adjusted for age, sex, BMI, smoking, hyperlipidemia, hypertension, diabetes, and other metals were performed. Crude and adjusted odds ratios (OR) with 95% confidence intervals (CI) were calculated. A one-way analysis of variance (ANOVA) was used to compare the blood heavy metal levels of study population by hypertension and diabetes, and analyzed the polynomial linear P for trend. P values<0.05 were considered statistically significant. All statistical analyses were performed using SPSS PASW statistics 18.0 (IBM, Armonk, NY, USA).
The overall analysis included 1,797 adults in the KNHANES, and of these subjects, 128 adults (7.1%) had CKD. A greater proportion of adults with hypertension, diabetes, hyperlipidemia, or high BMI had CKD (Table 1). The means of blood Pb, Hg, and Cd were 2.37±1.02 µg/dL, 4.35±3.33 µg/L, and 1.17±0.68 µg/L, respectively. The cut-off levels for blood Pb, Hg, and Cd level set by the World Health Organization (WHO) are 21 µg/dL, 5 µg/L and 5 µg/L. respectively. No paticipant was above the cut off levels of the WHO guidelines. Average blood Pb, Hg, and Cd levels were higher for subjects with CKD than for subjects without CKD (Table 1).
In the correlation analysis, we found that exposure to environmental heavy metals was correlated significantly with eGFR (Pb; r=-0.19, Hg; r=-0.07, Cd; r=-0.13, P<0.001) but only Cd was correlated with albumin creatinine ratio (ACR) (r=0.52, P=0.028). In univariate logistic analysis, blood Pb and Cd levels were associated with CKD. After multiple regression analysis adjusting for age, sex, BMI, smoking, hyperlipidemia, hypertension, diabetes, and other metals (multivariate model 3), blood Pb (OR, 1.05; 95% CI, 0.85-1.30, P=0.644), Hg (OR, 1.02; 95% CI, 0.97-1.07, P=0.479), and Cd level (OR, 1.09; 95% CI, 0.81-1.47, P=0.577) had no association with CKD (Table 2).
Next, we performed a stratified analysis for diabetes and for hypertension, which are major risk factors for CKD (Table 1). There were also associations between blood Cd level and CKD in hypertensive adults (n=499) by multiple logistic regression analysis (multivariate model 3) (OR, 1.52; 95% CI, 1.05-2.19, P=0.026), but there was no association between CKD and blood Pb and Hg level (Table 3). Diabetic adults (n=178) had a positive association between elevated blood Cd and CKD by multiple logistic regression analysis (multivariate model 3) (OR, 1.92; 95% CI, 1.14-3.25, P=0.014), but there was no association between CKD and blood Pb and Hg level (Table 4). Adults who were normotensive or who were non-diabetic had no association between the environmental heavy metals and CKD (Tables 3, 4). In ANOVA, blood Cd level showed significantly increasing trend in KNHANES participants according to hypertension and diabetes (Table 5).
The Korea Center for Disease Control and Prevention (KCDC) released a health report showing that blood Hg and Cd levels of Koreans are higher than those of people in other developed countries and that blood Pb levels are similar (2011 KNHANES report). The reason may be that Koreans live with more severe environmental pollution from cars, factories, and pesticides, and may ingest more contaminated seafoods and Chinese herbal medications. Therefore, there is a high probability of heavy metal toxicity from contamination of the air, soil, and water. Pb, Hg, and Cd are widespread environmental heavy metal toxicants. Environmental sources of Pb exposure include gasoline, contamination of food during processing, and contamination of air, soil, and water in areas close to old mines or garages (3, 4, 6, 7). Common sources of Hg include freshwater fish from polluted waters (3, 6). Sources of Cd exposure in the general population are cigarette smoke, ingestion of polluted vegetables, and breathing air from fuel combustion (2, 5, 6). In the human body, Cd accumulates in the renal cortex where more than 50% of the body burden is stored, and Pb accumulates in bone which contains about 90% of the body burden in adults (12). The biologic half-lives of these metals are on the order of decades. Because of these long half-lives (i.e., Cd more than 30 yr), prolonged, low level exposure can lead to excessive accumulation in certain tissues, especially the kidney, and this can perturb physiological function. Blood Cd levels are usually indicative of recent exposure, but also correlates well with total body Cd load (3, 6) and urine Cd (13). Blood Pb reflects exposure from current exogenous sources and also the release of endogenous Pb from bone (3, 6). Threfore, the blood concentrations of these heavy metals have been used as markers of chronic environmental exposure in the large population.
In the National Health and Nutrition Examination Survey (NHANES), blood Pb and Cd levels below the accepted thresholds were associated with low eGFR, albuminuria, or CKD (12, 14, 15, 16). In a Swedish population exposed to Cd, exposure to low environmental Cd appeared to be a determinant for the development of end-stage renal failure (ESRD) (17). In a population-based prospective nested case-referent study of low level exposure to environmental Pb, Hg, and Cd, erythrocyte Pb was associated with ESRD (18). However, in a longitudinal observation of the Cadmibel study, increased Cd burden was not associated with progressive renal dysfunction (19). In a recent review examining chronic renal failure and lead exposure, the kidney was not an early victim after lead exposure (7). Pb, Hg, and Cd at high levels in occupational exposures are known to be nephrotoxic, but the relevance of renal damage at low-level environmental exposures in the general population is controversial and the associations according to other CKD risk factors remain uncertain. The association between CKD and environmental toxin exposure has been studied exclusively in Western countries, and little data are available in Asian populations. In addition, there are few studies on the associations and effects according to diabetes or hypertension. In our results, environmental low dose esposures of Pb, Hg, and Cd were not associated with CKD after adjustment. Instead, in adults with hypertension or diabetes, CKD had a significant association with elevated blood Cd, but no association was present with blood Pb and Hg. Therefore, we suggest that Cd is a potential and important contributor to the epidemic of CKD and has a harmful effect on the kidneys in those who have previously suffered from diabetes or hypertension due to a synergic effect.
Previously published studies (20, 21) have suggested that blood Cd and Pb have been implicated in hypertension, even at low levels of exposure. Navas-Acien et al. (22) reported that blood Pb and Cd, at levels below current safety standards, were associated with an increased prevalence of peripheral arterial disease. Muntner et al. (14) reported the existence of an association between low levels of Pb exposure and CKD in hypertensive patients, but not in normotensive patients. Akesson et al. (23) reported that Cd-potentiated diabetes induced effects on the kidney. Although the effects were small, the tubular kidney effect occurred at lower Cd levels, especially in subjects with diabetes. Evans et al. (24) recently reported that there was no evidence of an important role for low-level occupational Pb exposure in the cause or progression of severe CKD. There is much debate about the relationship between low levels of environmental heavy metals and renal damage. Although environmental heavy metal exposures, especially Cd, in Korea are more common than in other developed countries, kidney toxicities are not more severe in our results. Several possible reasons for this discrepency are suggested by the results of the current study. Genetic factor, lifestyle pattern and low burden of other risk factors may be confounding factors (24, 25, 26, 27). Some Korean diet (i.e. greater consumption of vegetables, soy bean, garlic extract, and Korean red ginseng) might be a possible reason for the discrepency. There are some interesting experimental studies suggesting that garlic extracts have a reno-protective effect against Pb-induced hypertension and oxidative stress in an experimental rat model, but this should be further evaluated (28, 29).
The present findings should be interpreted in light of potential limitations. First, since the KNHANES is a cross-sectional survey, it is not possible to infer causality between elevated blood metals and CKD. The lack of prospective follow-up limits the ability to make inferences regarding the causality of the associations detected. In addition, reverse causation cannot be excluded as an explanation for our results. Second, our findings could also be limited by difficulties in fully adjusting for potential confounders, including socioeconomic status, food intake, or other environmental factors. Third, we assessed exposure to environmental heavy metal toxins by blood heavy metal level. Blood metal level is the most commonly used measure of chronic environmental exposure in general population studies, but blood Pb, Hg, and Cd levels may not be exact tissue levels and may also be considered to be markers of acute, short term exposure (30).
The main strength of this study is the evaluation of the effect of environmental exposure of Cd on CKD in real-life patients in a nationwide setting, using the well-characterized KNHANES datasets, which are characterized by a large sample size, a rigorous study protocol, high quality and extensive quality control, standardized laboratory procedures, and are representative of the Korean noninstitutionalized, civilian population. Since several potential confounders were collected as part of the KNHANES, we were able to examine the association between common environmental metals exposure and CKD, providing sufficient power to examine effect modification and adjust for a variety of sociodemographic and traditional kidney disease risk factors. Our results indicate an environmental low level of Pb, Hg, Cd exposure in the general population was not associated with CKD. However, low levels of Cd exposure may be harmful to kidney function, especially chronic environmental exposure in adults with hypertension or diabetes. Finally, given the high prevalence of hypertension and diabetes among persons with CKD, further prospective, large sample studies are needed to evaluate a causal relationship between environmental exposure to Cd and CKD in relation to hypertension and diabetes.
Our data have suggested that although the effects are weak for environmental low level exposures, environmental Cd exposure can cause vascular or renal damage, especially in diabetic or hypertensive subjects. Given the widespread and correlated exposures to the Cd and the increasing worldwide burden of CKD, these data have substantial public health implications for the general population. This risk may contribute to a synergic effect in the development and progression of CKD, especially in susceptible individuals with hypertension or diabetes.
Figures and Tables
Table 1
Characteristics of the 1,797 adults in the KHNANES (Korean National Health and Nutrition Examination Survey), a general population-based survey

Table 2
Multiple logistic regression analysis for CKD by blood lead (Pb), mercury (Hg), and cadmium (Cd) in the 1,797 adults in the KHNANES
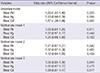
Table 3
Multiple logistic regression analysis for CKD by blood lead (Pb), mercury (Hg), and cadmium (Cd) according to hypertension
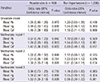
Table 4
Multiple logistic regression analysis for CKD by blood lead (Pb), mercury (Hg), and cadmium (Cd) according to diabetes
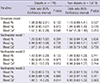
Table 5
Blood lead (Pb), mercury (Hg), and cadmium (Cd) levels in KNHANES participants according to hypertension and diabetes

ACKNOWLEDGEMENTS
The authors would like to thank to the staffs of KNOW-CKD study (Korean CKD cohorts) and KNHANES (https://knhanes.cdc.go.kr/knhanes/index.do).
References
1. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012; 379:165–180.
2. Järup L. Cadmium overload and toxicity. Nephrol Dial Transplant. 2002; 17:35–39.
3. Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003; 68:167–182.
4. Ekong EB, Jaar BG, Weaver VM. Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int. 2006; 70:2074–2084.
5. Johri N, Jacquillet G, Unwin R. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals. 2010; 23:783–792.
6. Soderland P, Lovekar S, Weiner DE, Brooks DR, Kaufman JS. Chronic kidney disease associated with environmental toxins and exposures. Adv Chronic Kidney Dis. 2010; 17:254–264.
7. Evans M, Elinder CG. Chronic renal failure from lead: myth or evidence-based fact? Kidney Int. 2011; 79:272–279.
8. Prozialeck WC, Edwards JR, Woods JM. The vascular endothelium as a target of cadmium toxicity. Life Sci. 2006; 79:1493–1506.
9. Thijssen S, Maringwa J, Faes C, Lambrichts I, Van Kerkhove E. Chronic exposure of mice to environmentally relevant, low doses of cadmium leads to early renal damage, not predicted by blood or urine cadmium levels. Toxicology. 2007; 229:145–156.
10. Edwards JR, Prozialeck WC. Cadmium, diabetes and chronic kidney disease. Toxicol Appl Pharmacol. 2009; 238:289–293.
11. Li SJ, Zhang SH, Chen HP, Zeng CH, Zheng CX, Li LS, Liu ZH. Mercury-induced membranous nephropathy: clinical and pathological features. Clin J Am Soc Nephrol. 2010; 5:439–444.
12. Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol. 2009; 170:1156–1164.
13. Olsson IM, Bensryd I, Lundh T, Ottosson H, Skerfving S, Oskarsson A. Cadmium in blood and urine--impact of sex, age, dietary intake, iron status, and former smoking--association of renal effects. Environ Health Perspect. 2002; 110:1185–1190.
14. Muntner P, He J, Vupputuri S, Coresh J, Batuman V. Blood lead and chronic kidney disease in the general United States population: results from NHANES III. Kidney Int. 2003; 63:1044–1050.
15. Ferraro PM, Costanzi S, Naticchia A, Sturniolo A, Gambaro G. Low level exposure to cadmium increases the risk of chronic kidney disease: analysis of the NHANES 1999-2006. BMC Public Health. 2010; 10:304.
16. Spector JT, Navas-Acien A, Fadrowski J, Guallar E, Jaar B, Weaver VM. Associations of blood lead with estimated glomerular filtration rate using MDRD, CKD-EPI and serum cystatin C-based equations. Nephrol Dial Transplant. 2011; 26:2786–2792.
17. Hellstrom L, Elinder CG, Dahlberg B, Lundberg M, Järup L, Persson B, Axelson O. Cadmium exposure and end-stage renal disease. Am J Kidney Dis. 2001; 38:1001–1008.
18. Sommar JN, Svensson MK, Björ BM, Elmståhl S, Hallmans G, Lundh T, Schön SM, Skerfving S, Bergdahl IA. End-stage renal disease and low level exposure to lead, cadmium and mercury; a population-based, prospective nested case-referent study in Sweden. Environ Health. 2013; 12:9.
19. Hotz P, Buchet JP, Bernard A, Lison D, Lauwerys R. Renal effects of low-level environmental cadmium exposure: 5-year follow-up of a subcohort from the Cadmibel study. Lancet. 1999; 354:1508–1513.
20. Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease--a systematic review. Environ Health Perspect. 2007; 115:472–482.
21. Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E. Cadmium exposure and hypertension in the 1999-2004 National Health and Nutrition Examination Survey (NHANES). Environ Health Perspect. 2008; 116:51–56.
22. Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004; 109:3196–3201.
23. Akesson A, Lundh T, Vahter M, Bjellerup P, Lidfeldt J, Nerbrand C, Samsioe G, Strömberg U, Skerfving S. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect. 2005; 113:1627–1631.
24. Evans M, Fored CM, Nise G, Bellocco R, Nyrén O, Elinder CG. Occupational lead exposure and severe CKD: a population-based case-control and prospective observational cohort study in Sweden. Am J Kidney Dis. 2010; 55:497–506.
25. Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, et al. Chronic Renal Insufficiency Cohort (CRIC) Study Group. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009; 4:1302–1311.
26. Hwang SJ, Tsai JC, Chen HC. Epidemiology, impact and preventive care of chronic kidney disease in Taiwan. Nephrology (Carlton). 2010; 15:3–9.
27. Hippisley-Cox J, Coupland C. Predicting the risk of chronic Kidney Disease in men and women in England and Wales: prospective derivation and external validation of the QKidney Scores. BMC Fam Pract. 2010; 11:49.
28. Hassan HA, El-Agmy SM, Gaur RL, Fernando A, Raj MH, Ouhtit A. In vivo evidence of hepato- and reno-protective effect of garlic oil against sodium nitrite-induced oxidative stress. Int J Biol Sci. 2009; 5:249–255.
29. Kilikdar D, Mukherjee D, Mitra E, Ghosh AK, Basu A, Chandra AM, Bandyoapdhyay D. Protective effect of aqueous garlic extract against lead-induced hepatic injury in rats. Indian J Exp Biol. 2011; 49:498–510.
30. Hu H, Rabinowitz M, Smith D. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect. 1998; 106:1–8.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download