Abstract
Figures and Tables
Table 1

*Values are expressed as mean±SD or median and 25% percentile-75% percentile or number and percent; †Using the t-test or chi-square test; ‡≥400 (20 pack/whole year); §≥3 times/week and ≥30 min/time. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; HOMAIR, homeostasis model for insulin resistance; UACR, urinary albumin creatinine ratio; BaPWV, brachial-ankle pulse wave velocity; DM, diabetes mellitus.
Table 2

*Valuesare expressed as mean±SD for age or mean±SE or median and 25% percentile-75% percentile except age or number and percent; †baPWV values were divided into4 quartiles: <1,379, 1,379-1,557, 1,557-1,796, >1,796 cm/s in men, <1,285, 1,285-1,489, 1,489-1,724, >1,724 cm/s in women; ‡Using a generalized linear model for continuous variables and the Cochran-Mantel-Haenzsel test for categorical variables adjusted for age; §≥400 (20 pack/whole year); ∥≥3 times/week and ≥30 min/time. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; HOMAIR, homeostasis model for insulin resistance; UACR, urinary albumin creatinine ratio; BaPWV, brachial-ankle pulse wave velocity; DM, diabetes mellitus.
Table 3

*Valuesexpressed as mean±SD or median and 25% percentile-75% percentile or number and percent; †UACR 30-300 mg/g; ‡Using t-test or chi-square test; §≥400 (20 pack/whole year); ∥≥3 times/week and ≥30 min/1 time; ¶baPWV values were divided into4 quartiles: <1,379, 1,379-1,557, 1,557-1,796, >1,796 cm/s in men, <1,285, 1,285-1,489, 1,489-1,724, >1,724 cm/s in women. MAU, microalbuminuria; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; HOMAIR, homeostasis model for insulin resistance; UACR, urinary albumin creatinine ratio; BaPWV, brachial-ankle pulse wave velocity; DM, diabetes mellitus.
Table 4

*Using multiple logistic regression; †Systolic blood pressure≥140 mmHg or diastolic blood pressure≥90 mmHg or medication of antihypertensive drugs; ‡Fasting blood glucose ≥126 mg/dL or history of diabetes mellitus or medication for diabetes mellitus; §BaPWV values were divided into4 quartiles: <1,379, 1,379-1,557, 1,557-1,796, >1,796 cm/s in men, <1,285, 1,285-1,489, 1,489-1,724, >1,724 cm/s in women. BaPWV, brachial-ankle pulse wave velocity; HOMAIR, homeostasis model for insulin resistance; HDL, high density lipoprotein.
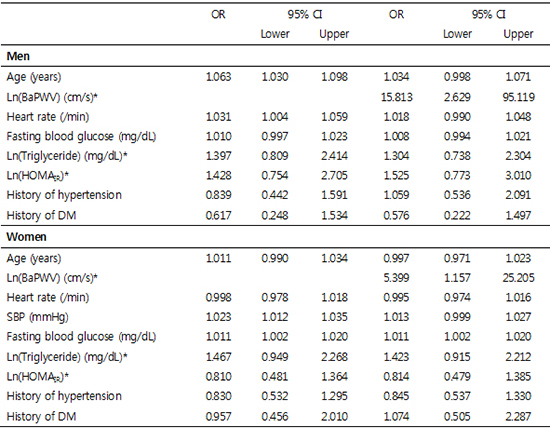
Table 5

Model I was adjusted for age (yr), pulse rate (/min), fasting blood glucose (mg/dL), log transformation triglyceride (mg/dL), log transformation HOMAIR, history of hypertension (yes/no) and history of DM (yes/no) in men; Model I was adjusted for age (yr), pulse rate (/min), systolic blood pressure (mmHg), fasting blood glucose (mg/dL), log transformation triglyceride (mg/dL), log transformation HOMAIR, history of hypertension (yes/no) and history of DM (yes/no) in women; Model II was adjusted for age (yr), log transformation baPWV (cm/s), pulse rate (/min), log transformation triglyceride (mg/dL), log transformation HOMAIR; Model III was adjusted for age (yr), log transformation baPWV (cm/s), pulse rate (/min), fasting blood glucose (mg/dL), log transformation triglyceride (mg/dL), log transformation HOMAIR; Model IV (yes/no) was adjusted for age (years), pulse rate (/min), log transformation baPWV (cm/s), fasting blood glucose (mg/dL), log transformation triglyceride (mg/dL), log transformation HOMAIR, history of hypertension (yes/no) and history of DM (yes/no); Model V was adjusted for age (yr), log transformation baPWV (cm/s), pulse rate (/min), fasting blood glucose (mg/dL), log transformation triglyceride (mg/dL), log transformation HOMAIR, history of hypertension (yes/no) and history of DM (yes/no) in men; Model V was adjusted for age (yr), log transformation baPWV (cm/s), pulse rate (/min), systolic blood pressure (mmHg), fasting blood glucose (mg/dL), log transformation triglyceride (mg/dL), log transformation HOMAIR, history of hypertension (yes/no) and history of DM (yes/no) in women. *Log transformation was done to obtain a normal distribution. BaPWV, brachial-ankle pulse wave velocity; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMAIR, homeostasis model for insulin resistance; DM, diabetes mellitus.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download