Abstract
Novel agents to treat multiple myeloma (MM) have increased complete respone (CR) rates compared with conventional chemotherapy, and the quality of the response to treatment has been correlated with survival. The purpose of our study was to show how of early response to bortezomib combined chemotherapy influences survival in patients with newly diagnosed MM who are ineligible for stem cell transplantation. We assessed patient responses to at least four cycles of bortezomib using the International Myeloma Working Group response criteria. The endpoints were comparisons of progression free survival (PFS) and overall survival (OS) between early good response group (A group) and poor response group (B group). We retrospectively analyzed data from 129 patients registered by the Korean Multiple Myeloma Working Party, a nationwide registration of MM patients. The 3 yr PFS for the A and B groups was 55.6% and 18.4%, respectively (P < 0.001). The 3 yr OS for the A and B groups was 65.3% and 52.9%, respectively (P = 0.078). The early response to at least four cycle of bortezomib before next chemotherapy may help predict PFS in patients with MM who are ineligible stem cell transplantation.
Multiple myeloma (MM) patients treated with conventional chemotherapies, such as melphalan and prednisone (MP) or vincristine, doxorubicin, and dexamethasone (VAD), do not commonly have a complete response (CR) (1-4). Novel agents for treating MM, such as thalidomide, bortezomib, and lenalidomide, generally result in an improved response and longer survival. Patients undergoing high dose chemotherapy (HDT) plus autologous stem cell transplantation (ASCT) and regimens incorporating bortezomib, thalidomide, and lenalidomide have better responses than patients undergoing conventional chemotherapy (5-8). Moreover, the quality of a response to a MM treatment has been correlated with survival. A CR to induction therapy was reported to be a predictive factor of outcome for patients undergoing autologous stem cell transplantation (9, 10). Moreover, a CR or very good partial response (VGPR) was also associated with longer survival in patients not undergoing HDT or ASCT (11-14). Especially in elderly patients, an early response to therapy was associated with survival in patients with MM (15-17). Patients with a ≥ 50% decrease in monoclonal protein after 1 cycle of vincristine, doxorubicin and dexamethasone had a better event free survival (EFS) than patients with a < 50% reduction (15). Another report showed a survival advantage for patients with a decrease in M-protein of 30% or more after 1 MP cycle (17). In elderly patients, a treatment can increase the CR rate without improving PFS because of a higher toxic death rate, poor performance status, and poor compliance with intensive therapy or aggressive treatment (18, 19). Bortezomib combined chemotherapy, however, is effective and tolerable in elderly patients with MM (20).
Additionally, a relationship between a rapid response and outcomes has been demonstrated in patients with relapsed and/or refractory MM who were treated with bortezomib and pegylated liposomal doxorubincin (21). Patients with a rapid response had a higher survival rate. To date, there is no data on the relationship between an early response and survival in patients treated with novel agents, including bortezomib combined chemotherapy as first-line therapy. The purpose of this study is to show the relationship between an early response and survival in MM patients treated with bortezomib combined chemotherapy.
Patients who were newly diagnosed with MM and underwent bortezomib combined chemotherapy as first-line therapy between September 2003 and July 2011 were analyzed retrospectively in this study. Data were collected through the Korean Multiple Myeloma Working Party (KMMWP), a nationwide registry of MM patients. The total number of patients who were treated with bortezomib was 1,176 in Korea. However, bortezomib was given to only 204 patients as first-line treatment, among which 75 received autologous stem cell transplantation (ASCT) and 129 did not receive ASCT. The reason of small number of patients treated with first-line bortezomib compared with the total number is that bortezomib was approved only for those who had failed from first-line chemotherapy by the Health Insurance Review Agency in Korea.
We included patients who were ineligible for hematopoietic stem cell transplantion (HSCT) because of their age (65 yr or older) or comorbidities. All enrolled patients were treated with at least four cycles of bortezomib combined chemotherapy. Patients who received HSCT or had other malignancies were excluded.
In this study, there were 4 different regimens of bortezomib combined chemotherapy. Fifty-seven patients (44.2%) were treated with bortezomib plus dexamethasone (VD), comprising an intravenous bolus of bortezomib (1.3 mg/m2) on days 1, 4, 8, and 11, an oral or intravenous bolus of dexamethasone (20 mg fixed dose) on days 1-2, 4-5, 8-9, and 11-12, every 3 weeks. Thirty-two patients (24.8%) were treated with bortezomib, thalidomide, and dexamethasone (VTD), comprising an intravenous bolus of bortezomib (1.3 mg/m2) on days 1, 4, 8, and 11, oral thalidomide (100 mg) daily, and an oral or intravenous bolus of dexamethasone (40 mg) on days 1 to 4, every 3 weeks. Eighteen patients (14.0%) were treated with doxorubicin, bortezomib, and dexamethasone (PAD), comprising an intravenous bolus of bortezomib (1.3 mg/m2) on days 1, 4, 8, and 11, an intravenous bolus of doxorubicin (9 mg/m2) on days 1 to 4, an oral or intravenous bolus of dexamethasone (40 mg fixed dose) on days 1 to 4, every 3 weeks. Twenty-two patients (17.1%) were treated with bortezomib, melphalan, and prednisone (VMP), comprising an intravenous bolus of bortezomib (1.3 mg/m2) on days 1, 4, 8, 11, 22, 25, 29, and 32, cycles 1 to 4, and days 1, 8, 22, and 29, cycles 5 to 9, oral melphalan (9 mg/m2) on days 1 to 4, cycles 1 to 9, and oral prednisone (60 mg/m2) on days 1 to 4, cycles 1 to 9, every 6 weeks.
International Myeloma Working Group response criteria (22) was used to assess responses in all patients receiving bortezomib combined chemotherapy. To determine the early response, patients were assessed before cycle 4 or 4 months after starting chemotherapy.
A stringent complete response (sCR) was defined as a normal free light chain (FLC) ratio and an absence of clonal cells in bone marrow by immunohistocheminstry or immunofluorescence. CR was defined as negative immunofixation on the serum and urine, disappearance of any soft tissue plasmacytoma, and less than 5% plasma cells in bone marrow. A very good partial response (VGPR) was defined as, 1) serum and urine M-protein detectable by immunofixation but not electrophoresis, or 90% or greater reduction in serum M-protein, plus 2) urine M-protein level less than 100 mg per 24 hr. Partial response (PR) was defined as a 50% or greater reduction in serum M-protein and reduction in 24 hr urinary M-protein of 90% or more or to less than 200 mg. Progressive disease (PD) was defined as a relative increase of 25% or greater or an absolute increase of 0.5 g/dL or more in serum or urine M-component or 200 mg or more per 24 hr in urine. Stable disease (SD) was defined as not meeting criteria for CR, VGPR, PR or PD.
For comparison patients were grouped as VGPR and CR or PR, SD, and PD. All patients were divided into these two groups based on incidence rates of their early response before cycle 4 or 4 months after starting chemotherapy. The incidence rates of patients with a VGPR or better, and a PR or worse were 51.2% and 48.8%, respectively. To compare categorical variables between the two groups chi-square test was done. OS was defined as the time from initiating the study therapy to date of death from any cause. PFS was defined as the time from initiating the study therapy to date of progressive disease (PD). Patients who died without documented PD were considered to have had PD at the time of death. Patients lost to follow-up were censored at the last contact date. Survival curves were estimated using the Kaplan-Meier method.
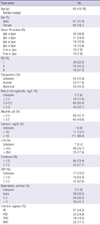
The median patient age was 63 yr (range from 45 to 76). The male to female ratio was 1.08:1.00. The median follow up duration from the diagnosis to last follow up date was 20.73 months (range from 4.33 to 80.23 months). The median duration from the first chemotherapy to evaluation of early response was 2.3 months (range from 0.9 to 3.7 months). Other patient characteristics are shown in Table 1.
All patients were divided into two groups according to their early response. Patients with a VGPR or CR composed the A group, and patients with a PR, SD, or PD composed the B group. All baseline and treatment related factors were similar between the two groups, except treatment regimen and additional chemotherapy after first line chemotherapy. Of the A group, 34.8% were treated with VD and 36.4% were treated with VTD; 54.0% of the B group was treated with VD. Additional chemotherapies were given more frequently in group B than those in group A, as shown in Table 2.
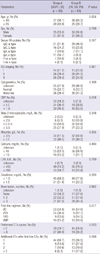
The response results after chemotherapy are shown in Table 3. The early response was CR for 16 patients (12.4%), VGPR for 50 (38.8%), PR for 40 (31.0%), SD for 14 (10.9%) and PD for 9 (7.0%). The best response was CR for 46 patients (35.7%), VGPR for 31 (24.0%), PR for 32 (24.8%), SD for 11 (8.5%) and PD for 9 (7.0%).
Table 4 shows a comparison of PFS and OS between the A and B groups. The 3 yr PFS were higher in the A group than the B group (55.6% vs 18.4%, P < 0.001). The 3 yr OS tended to be higher in the A group than the B group, though the difference was not statistically significant (65.3% vs 52.9%, P = 0.078), (Fig. 1).
PFS and OS were compared between early responders and delayed responders. The early responders (n=66) included patients who had a VGPR or CR at an early time point. The delayed responders (n=11) included patients who have a VGPR or CR after the fourth cycle or four months of chemotherapy. The 3 yr PFS was higher among early responders than delayed responders (55.6% vs 33.3%, P = 0.031). The 3 yr OS did not differ between the groups (65.3% vs 75.0%, P = 0.831) (Fig. 2).
We next analyzed the effect of each chemotherapy regimens on PFS and OS. The 3 yr PFS was significantly higher for the A group when treated with VD, VTD, or PAD (VD; P = 0.002, VTD; P = 0.001, PAD; P = 0.002, and VMP; P = 0.119). The 3 yr OS was only significantly higher for the A group treated with PAD (VD; P = 0.215, VTD; P = 0.240, PAD; P = 0.047, and VMP; P = 0.345). Though the differences were not significant, the early responders tended to have better outcomes than the delayed responders when stratified by chemotherapeutic regimen.
On further analysis, PFS and OS were compared between group A and B according to International Staging System (ISS). In ISS I and II, The 3 yr PFS were higher in the A group than the B group (P < 0.001 and P < 0.001). The 3 yr OS tended to be higher in the A group than the B group, though the difference was not statistically significant (P = 0.718 and P = 0.182). However, in ISS III, The 3 yr PFS and OS were higher in the A group than the B group (P < 0.001 and P < 0.042).
In some studies, achieving a CR (or the maximal response) has been associated with the long-term outcome of MM patients who were ineligible for stem cell transplantation (11-13, 23, 24). The addition of bortezomib, thalidomide, and lenalidomide to first-line therapies for nontransplant MM patients has resulted in high CR and VGPR rates in some pahse III studies (19, 23, 25). The VISTA trial, in which bortezomib plus MP (VMP) was compared with MP alone in terms of CR or VGPR versus PR, demonstrated that VMP was associated significantly with a longer time to progression (TTP, P = 0.025), a longer time to next therapy (TTNT, P = 0.005) and a longer treatment-free interval (TFI, P = 0.002), but not with longer OS (P = 0.54).
There have been some reports that the early response is predictive of the therapeutic coutcome. Schaar and colleagues (17) studied the relationship between survival and the rate of M protein decrease during the first cycles of therapy in newly-diagnosed MM patients. The survival advantage was seen for patients who had an M protein decrease of at least 30%, indicating that an early response to MP predicted survival in MM. Ross et al. (15) demonstrated that patients with a ≥50% reduction in monoclonal protein after the first cycles of VAD had a significantly better EFS than patients with <50% reduction (P = 0.002). A recent report by Shah and colleagues (21) focused on patients with relapsed or refractory MM treated with a novel agent. The study showed that patients with a 50% or greater, and especially those with a 75% or greater, reduction in M protein levels at cycle 2 had a significant decreased risk for TTP, compared with patients with less than a 25% reduction. Thus early decreases in M protein may provide better outcomes in patients treated with bortezomib combined chemotherapy. Palumbo (16) also reported the possibility of using a response marker in the early phases of therapy to predict outcome, but until now there have been no data about the early response in MM patients who were not eligible for HSCT treated with novel agents as first-line therapy.
In our study, all patients, who were treated with bortezomib combined chemotherapy, were divided into a good response group, which included patients with a VGPR or better, and a poor response group, which included patients with a PR or worse. The groups were determined based on patient responses before cycle 4 or 4 months after starting chemotherapy. The 3 yr PFS of the good response group was higher than the poor response group (P < 0.001), though the 3 yr OS did not differ (P = 0.078). No significant differences of OS between two groups might be influenced by more numbers of chemotherapy after first line chemotherapy in group B than those in group A in based of patients characteristics. Moreover, within the good response group, patients with an early response had significantly higher survival rates than patients with a delayed response. The 3 yr PFS was higher in the early response group than the delayed response group (P = 0.031), but the 3 yr OS did not differ (P = 0.831).
This study has several limitations. We used retrospective medical record review at the nationwide registry of MM patients. More research is needed to investigate the effect of early responses to novel agents on MM outcomes.
In summary, our results suggest that patients that have a early good response to bortezomib combined chemotherapy as first-line therapy have longer PFS than those that have a poor response by cycle 4 or 4 months after starting chemotherapy. An early response may be predictive for survival outcomes in nontransplant candidate patients with MM treated with bortezomib combined chemotherapy.
Figures and Tables
Fig. 1
Comparison of progression free survival (PFS) and overall survival (OS) rates between the early good and poor response groups. The early good response group has a higher PFS (P < 0.001) (A). The early good response group tends to have a higher OS (P = 0.0078) (B). CR, complete response; VGPR, very good partial response; PR, partial response; SD, stable disease; PD, progressive disease.

Fig. 2
Comparison of progression free survival (PFS) and overall survival (OS) rates between the early and delayed response groups. The early response group has a higher PFS (P = 0.031) (A). There is shown no difference between the groups in terms of OS (P = 0.831) (B). CR, complete response; VGPR, very good partial response; PR, partial response; SD, stable disease; PD, progressive disease.

Table 4
Survival rates after chemotherapy

CR, complete response; VGPR, very good partial response; PR, partial response; SD, stable disease; PD, progressive disease; PFS, progression free survival rates; OS, overall survival rates, early response, more than PR at least four cycles or less than 4 months; delayed response, more than PR after four cycles or 4 months.
References
1. Facon T, Mary JY, Pegourie B, Attal M, Renaud M, Sadoun A, Voillat L, Dorvaux V, Hulin C, Lepeu G, et al. Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood. 2006. 107:1292–1298.
2. Hernandez JM, Garcia-Sanz R, Golvano E, Blade J, Fernandez-Calvo J, Trujillo J, Soler JA, Gardella S, Carbonell F, Mateo G, et al. Randomized comparison of dexamethasone combined with melphalan versus melphalan with prednisone in the treatment of elderly patients with multiple myeloma. Br J Haematol. 2004. 127:159–164.
3. Alexanian R, Barlogie B, Tucker S. VAD-based regimens as primary treatment for multiple myeloma. Am J Hematol. 1990. 33:86–89.
4. Segeren CM, Sonneveld P, vanderHolt B, Baars JW, Biesma DH, Cornellissen JJ, Croockewit AJ, Dekker AW, Fibbe WE, Löwenberg B, et al. Vincristine, doxorubicin and dexamethasone (VAD) administered as rapid intravenous infusion for first-line treatment in untreated multiple myeloma. Br J Haematol. 1999. 105:127–130.
5. Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003. 348:1875–1883.
6. Lenhoff S, Hjorth M, Holmberg E, Turesson I, Westin J, Nielsen JL, Wisloff F, Brinch L, Carlson K, Carlsson M, et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Blood. 2000. 95:7–11.
7. Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist. 2007. 12:664–689.
8. Richardson PG, Mitsiades C, Schlossman R, Ghobrial I, Hideshima T, Munshi N, Anderson KC. Bortezomib in the front-line treatment of multiple myeloma. Expert Rev Anticancer Ther. 2008. 8:1053–1072.
9. Gertz MA, Kumar S, Lacy MQ, Dispenzieri A, Dingli D, Hayman SR, Buadi FK, Hogan WJ. Stem cell transplantation in multiple myeloma: impact of response failure with thalidomide or lenalidomide induction. Blood. 2010. 115:2348–2353.
10. Kim JS, Kim K, Cheong JW, Min YH, Suh C, Kim H, Jo DY, Ryoo HM, Yoon SS, Lee JH. Complete remission status before autologous stem cell transplantation is an important prognostic factor in patients with multiple myeloma undergoing upfront single autologous transplantation. Biol Blood Marrow Transplant. 2009. 15:463–470.
11. Kyle RA, Leong T, Li S, Oken MM, Kay NE, VanNess B, Greipp PR. Complete response in multiple myeloma: clinical trial E9486, an Eastern Cooperative Oncology Group study not involving stem cell transplantation. Cancer. 2006. 106:1958–1966.
12. Offidani M, Corvatta L, Piersantelli MN, Visani G, Alesiani F, Brunori M, Galieni P, Catarini M, Burattini M, Centurioni R, et al. Thalidomide, dexamethasone, and pegylated liposomal doxorubicin (ThaDD) for patients older than 65 years with newly diagnosed multiple myeloma. Blood. 2006. 108:2159–2164.
13. Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, Montanaro M, Ria R, Capaldi A, Zambello R, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008. 112:3107–3114.
14. Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, Dib M, Guillerm G, Salles B, Eschard JP, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009. 27:3664–3670.
15. Ross DM, To LB, Horvath N. Assessment of early paraprotein response to vincristine-doxorubicin-dexamethasone chemotherapy may help guide therapy in multiple myeloma. Intern Med J. 2004. 34:576–578.
16. Palumbo A. Early response predicts myeloma outcome. Blood. 2010. 115:2332–2333.
17. Schaar CG, Kluin-Nelemans JC, le Cessie S, Franck PF, te Marvelde MC, Wijermans PW. Early response to therapy and survival in multiple myeloma. Br J Haematol. 2004. 125:162–166.
18. Ludwig H, Hajek R, Tothova E, Drach J, Adam Z, Labar B, Egyed M, Spicka I, Gisslinger H, Greil R, et al. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2009. 113:3435–3442.
19. Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, Renaud M, Harousseau JL, Guillerm G, Chaleteix C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007. 370:1209–1218.
20. San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008. 359:906–917.
21. Shah J, Blade J, Sonneveld P, Harousseau JL, Lantz K, Londhe A, Lowery C, Orlowski RZ. Rapid early monoclonal protein reduction after therapy with bortezomib or bortezomib and pegylated liposomal doxorubicin in relapsed/refractory myeloma is associated with a longer time to progression. Cancer. 2011. 117:3758–3762.
22. Palumbo A, Sezer O, Kyle R, Miguel JS, Orlowski RZ, Moreau P, Niesvizky R, Morgan G, Comenzo R, Sonneveld P, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transplantation. Leukemia. 2009. 23:1716–1730.
23. Harousseau JL, Palumbo A, Richardson PG, Schlag R, Dimopoulos MA, Shpilberg O, Kropff M, Kentos A, Cavo M, Golenkov A, et al. Superior outcomes associated with complete response in newly diagnosed multiple myeloma patients treated with nonintensive therapy: analysis of the phase 3 VISTA study of bortezomib plus melphalan-prednisone versus melphalan-prednisone. Blood. 2010. 116:3743–3750.
24. Palumbo A, Falco P, Corradini P, Falcone A, DiRaimondo F, Giuliani N, Crippa C, Ciccone G, Omedè P, Ambrosini MT, et al. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA-Italian Multiple Myeloma Network. J Clin Oncol. 2007. 25:4459–4465.
25. Gay F, Vincent Rajkumar S, Falco P, Kumar S, Dispenzieri A, Petrucci MT, Gertz MA, Boccadoro M, Keith Stewart A, Palumbo A. Lenalidomide plus dexamethasone vs lenalidomide plus melphalan and prednisone: a retrospective study in newly diagnosed elderly myeloma. Eur J Haematol. 2010. 85:200–208.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download