Abstract
Lung cancer rarely occurs in young patients. Recent studies have demonstrated that epidemiologic data are closely correlated to some molecular characteristics. We investigated the clinicopathologic characteristics of lung adenocarcinoma in young patients and evaluated immunohistochemically detected epidermal growth factor receptor (EGFR) mutation status and anaplastic lymphoma kinase (ALK) positivity. Among lung adenocarcinoma patients, 31 cases were of the ≤ 40 yr-old group and 261 cases of > 50 yr-old group. Young patients were more likely to be females (67.7% vs 40.2%), and nonsmokers (58.1% vs 45.2%) and more often had high TNM stage (stage IV was 80.6% vs 52.1%) and had a high rate of distant metastasis (51.6% vs 28.0%) compared with older patients. The signet ring cell feature was more common (25.8% vs 11.5%) and lepidic growth pattern was rarely present (3.2% vs 16.5%) in the adenocarcinoma of young patients. There was no significant survival difference between the two age groups. The rate of EGFR mutation status and ALK positivity did not show a statistical difference between two groups. In conclusion, lung adenocarcinoma of young patients demonstrates distinct pathologic features with frequent presence of a signet ring cell feature and rare occurrence of lepidic growth pattern. Further investigation for other genetic abnormalities would be needed.
Lung cancer is a leading cause of cancer-related death worldwide including Korea, in which it has been estimated to affect 21.7% of all cancer-related deaths in 2009 (1). Lung cancer usually occurs in old-aged patients, and is the most common cancer in male patients older than 65 yr (1). It rarely affects young patients, and only about 1.1%-5% of all cases are seen in patients aged ≤ 40 yr (2-4). There are many studies about lung cancer arising in young patients in the English literature (2-8). According to these studies, young patients are more commonly female (2, 3, 5-7) and non-smokers (3, 4), who more often have adenocarcinoma with a lower incidence of squamous cell carcinoma (2, 3, 5, 6), more often present with advanced disease (2, 7), and who have better (2, 8) or similar overall survival rates (3, 6, 7) when compared with older patients. However, differences are present in the clinical and pathologic findings, and in the prognosis between studies about lung cancer from different times and regions.
Recent studies demonstrated that specific epidemiologic data and pathologic features are closely related to some molecular characteristics of lung adenocarcinoma, such as epidermal growth factor receptor (EGFR) and activation of the anaplastic lymphoma kinase gene (ALK) by fusion to echinoderm microtubule-associated protein-like 4 gene (EML4). EGFR mutations are frequently identified in females, nonsmokers, Asian ethnics (9, 10), and are related to a bronchioloalveolar growth pattern (11) or papillary and micropapillary features (12). Non-small cell lung cancers (NSCLC) having ALK rearrangement are associated with a younger age group, with non-smokers, with males, and have a signet ring cell morphology (13, 14).
As described above, predominantly females, nonsmokers and cases of adenocarcinoma histology are closely associated with young patients who have lung cancer, and thus we hypothesized that specific molecular and pathologic features might be associated with lung adenocarcinoma arising in young patients. Despite our research, most studies regarding lung cancer in young patients have focused on clinical characteristics, not on a thorough evaluation of pathologic features and molecular characteristics (2, 3, 5, 8). Furthermore, although a large proportion of young patients with lung adenocarcinoma presented at an advanced stage, the studies focusing on the pathologic features enrolled only surgically treated cases (15).
Immunohistochemistry (IHC) is a widely applicable technique in pathology laboratories for the analysis of even small biopsy samples or cytologic preparations which often struggle to provide sufficient amounts or high quality samples of DNA. Furthermore, IHC preserves tissue morphology and can detect a very small number of mutation-positive cancer cells even in the cases with overwhelming amounts of non-neoplastic cells. When using direct sequencing methods to detect EGFR mutation, small biopsy samples often do not provide enough DNA with a high enough quality to process, and mutations in tumor samples, in which the number of EGFR-mutant cancer cells is scant, are often obscured. Yu et al. (16) generated two mutation-specific antibodies that can specifically detect the two most common EGFR mutations in lung cancer, which represent 85%-90% of EGFR mutations in lung cancer; an L858R substitution mutation in exon 21 (L858R) and in-frame deletions in exon 19 (E746) (9). They showed that these antibodies are highly sensitive and specific with a sensitivity of 79%-95%, and a specificity of 99% for both antibodies, respectively (16, 17). Thus, an IHC assay with mutation-specific antibodies is a simple, rapid and reliable screening method to identify EGFR mutations especially in small biopsy tissue samples of lung cancer (16).
Fluorescent in situ hybridization (FISH) is a standard method to detect ALK rearrangement in lung cancer, but it is difficult to use FISH on all the biopsied or resected lung cancer samples because ALK rearrangement rate in NSCLC is very low (13) and the cost of FISH is very high. Paik et al. (14) demonstrated a good correlation between the results using a semi-quantitative scoring method with IHC and FISH for ALK rearrangement and reported the sensitivity and specificity at 100% and 95.8% respectively. Based on these findings, the IHC assay may be useful as a screening method to detect ALK-rearranged lung cancer.
In this study, we evaluated the clinicopathologic characteristics of lung adenocarcinoma of young patients and tested EGFR mutation status and ALK rearrangement using IHC in patients younger than 40 yr of age compared with those over 50 yr of age.
This study included 1,255 consecutive patients with primary lung cancer who underwent histologic diagnosis at Inha University Hospital, Korea, between 1998 and 2009. Cases with a history of other known malignancies or clinically diagnosed lung cancer cases without histologic confirmation were excluded. The patients were divided into three groups according to the age at diagnosis: a young age group encompassing patients who were 40 yr old or younger, an old age group encompassing patients who were older than 50 yr of age, and an intermediate age group encompassing the patients who were 41 to 50 yr old (inclusive). A total of 52 cases were categorized as the young age group, 1,095 cases comprised the old age group and 108 cases formed the intermediate age group. Because the patients aged between 41 and 50 yr showed common features of the other two age groups, we excluded the intermediate group on the statistical evaluations of the clinicopathologic differences between the young and old age groups. We reviewed patient's medical records to ascertain the clinical findings including age, sex, smoking history, histologic type of lung cancer, TNM stage, the time of diagnosis and death. We obtained their additional survival data from the Archives of the National Cancer Information Center. The stage of disease was defined according to the 7th edition of TNM cancer staging system approved by the American Joint Committee on Cancer/International Union against Cancer (18). Smoking status was divided into smokers (including current and previous smokers) vs non-smokers. A patient with a more than five pack-year history of smoking in his or her lifetime was defined as a smoker.
Among 416 cases of adenocarcinoma patients of the young and old age groups, only 292 cases of patients had available pathologic slides and sufficient tissue for IHC. Among 31 cases of the young age group, tissues were obtained through biopsies (including bronchoscopic biopsies, needle biopsies and incisional biopsies) in 24 cases and 7 cases had excisions performed (including an excisional biopsy, a wedge resection, a lobectomy and a pneumonectomy).The tissues were obtained in 21 cases from the lung including bronchus, in 6 cases from the pleura, and in 3 cases from the lymph nodes. In the old age group, 177 were biopsy cases whereas 84 were excision cases. The tissues were obtained from the lung in 207 cases, from the pleura in 22 cases, from the lymph nodes in 25 cases and from bone in 5 cases. We reviewed conventional pathologic slides and evaluated the pathologic diagnosis, predominant histologic pattern, tumor differentiation, presence of a micropapillary feature, lepidic growth pattern, and signet ring cell feature, presence of satellite nodules in the lung, and tumor location indicating whether the tumor was in the central region or in a peripheral area of the lung. If the case demonstrated that 5% or more of the tumor cells showed micropapillary feature, a lepidic growth pattern or signet ring cell feature, we categorized the case as a presence of each pattern. Signet ring cells were defined as cells distended with intracellular mucin droplets that displace the crescentic nuclei toward the edge of the cells based on the histologic criteria of the WHO classification in 2004 (19). The predominant histologic patterns were categorized as acinar predominant if a majority of tumor cells formed glands; as papillary predominant if a growth of glandular cells along the central fibrovascular cores was a major features; as lepidic predominant if the majority of tumor showed lepidic growth along the alveolar wall; micropapillary predominant if tumor cells grew in papillary tufts without fibrovascular core; and as solid predominant if polygonal tumor cells forming sheets without other recognizable patterns were predominant (20).
For the IHC study, we constructed tissue microarrays for 95 excisional cases. Samples with a diameter of 1.5-2 mm of the representative core tissues were taken from formalin fixed and paraffin embedded tissue blocks of excisional cases. Paraffin blocks of tissue microarray and other biopsy cases were serially sectioned at a 4 µm thickness and stained according to the previous descriptions (14, 16).
For the EGFR mutational assay, two rabbit monoclonal antibodies were used, one specific for the exon 21 L858R EGFR mutation (L858R) and the other for the 15bp, E746-750 deletion in exon 19 (E746)(43B2 and 6B6, Cell Signaling Technology, Danvers, MA, USA). An IHC was performed according to the following protocol: after overnight incubation at 55℃ and deparaffinization, antigen retrieval was performed using microwave boiling for 10 min in 1 mM/L EDTA. Intrinsic peroxidase activity was blocked by 3% hydrogen peroxide for 10 min. Diluted primary antibodies (1:100 dilution for both antibodies) were applied and the slides were incubated for 30 min. For detection, the EnVision kit (Dako) was used following the manufacturer's recommendations. Hematoxylin counterstain for 4 min was applied.
For the ALK rearrangement assay, the mouse monoclonal antibody for ALK (clone 5A4, Novocastra, Newcastle, United Kingdom) was used. Tissues were stained using a Ventana automated immunostainer (Ventana Medical Systems, Tucson, AZ, USA) according to the following protocol. The slides were dried at 60℃ for 1 hr and deparaffinized using EZ Prep (Ventana Medical Systems) at 75℃ for 4 min. The cells were pretreated with a CC1 solution containing Tris/borate/ethylenediamine tetraacetic acid at 100℃ for 20 min. The diluted primary antibody (1:30 dilution) was applied and incubated at 42℃ for one and a half hours. Signals were detected using an I-view detection kit (Ventana Medical Systems) based on the labeled streptavidin-biotin method. Hematoxylin counterstain was applied for 2 minutes at room temperature.
The IHC stained slides were independently reviewed and interpreted by two pathologists. The intensity of the staining and the percentage of positive cells were evaluated for the EGFR mutation assay. Slides were scored based on cytoplasmic and/or membranous staining intensity as follows: (0) when no staining or faint staining intensity was seen in ≤ 10% of the tumor cells; (1+) when faint staining was in more than 10% of the tumor cells; (2+) when moderate staining was observed in the tumor cells; (3+) when strong staining was observed in the tumor cells. Tumors with a score of (1+) to (3+) were interpreted as positive, and tumors with no expression (0), were regarded as negative (16, 17).
ALK IHC scores were assigned as follows: (0) if no stained cells were present; (1+) if faint or weak staining intensity was seen in more than 5% of the tumor cells or a moderate or strong staining intensity in ≤ 5% tumor cells; (2+) if moderate staining intensity was present in more than 5% of the tumor cells; (3+) if strong staining intensity was present in more than 5% of the tumor cells (13). We interpreted the assay as positive when tumors had a score of (2+) and (3+), and as negative when the score was (0) or (1+).
A Pearson's chi-squared test was used to determine the statistical significance of differences between the young and old age groups of total lung cancer in terms of sex, smoking history, histology type, and TNM stage. For lung adenocarcinoma, the differences within the following variables were evaluated between the young and old age groups. The variables included sex, smoking history, TNM stage, satellite nodules in the lung, tumor location, predominant histologic pattern, tumor differentiation, cellular features, presence of micropapillary feature, lepidic growth pattern, and signet ring cell feature, EGFR mutation status and ALK rearrangement. The Kaplan-Meier method with a log-rank test and Cox regression analysis were used for survival analysis. Overall survival time was calculated from the date of diagnosis of lung cancer to the date of death. Patients without a known date of death were censored at the time of the last follow-up. SPSS version 12.0 program (Systat, Chicago, IL, USA) was used for the Pearson's chi-squared test and survival analysis. Statistical significance was defined as P < 0.05.
Among the total of 1,255 primary lung cancer patients, 52 (4.9%) were aged ≤ 40 yr at the time of diagnosis. In the young age group, 24 (46.2%) were men and 28 (53.8%) were women (male-to-female ratio, 0.86:1). The age of these patients ranged from 25-40 yr with a mean age of 35.9 yr. Twenty-five (47.2%) were non-smokers in this group. In the old age group (n = 1,095), 855 (78.1%) were men and 240 (21.9%) were women (male-to-female ratio, 3.56:1). The age range was 51-90 yr with a mean age of 66.6 yr. Only 245 (22.5%) were non-smokers. The predominant histologic type of the lung cancer in young age group was adenocarcinoma (78.8%) and squamous cell carcinoma was rare (7.5%). Whereas, in the old age group, squamous cell carcinoma was more frequently present (n = 455, 41.6%) and 34.2% of patients (n = 375) were diagnosed with adenocarcinoma. In the young age group, the diagnosis of lung cancer was discovered at more advanced stages. Only 7 patients (13.5%) presented with TNM stage I or II disease, 7 (13.5%) presented with stage III and 36 (69.2%) were stage IV. In the old age group, 225 patients (20.5%) were at stage I and II of the disease, 339 (31.0%) were at stage III and only 279 (25.4%) were at stage IV (Table 1). In the young age group, a prevalence of females, nonsmokers, and an adenocarcinoma histology and at more advanced stages of the diagnoses were statistically different from those in the old age group. There was no significant difference of overall survival between the young and old age groups (P = 0.893) with a median survival time of 14 months and 13 months, respectively (Fig. 1A).
In the young age group with histologically confirmed primary lung adenocarcinoma, females were 67.7% (n = 21) and nonsmokers were 58.1% (n = 18). However, in the old age group, 40.2% (n = 105) and 45.2% (n = 118) were females and nonsmokers (P = 0.003 and 0.028, respectively). When compared with the old age group, the young age group tended to be in a more advanced stage at diagnosis. Stage IV found 80.6% (n = 25) vs 52.1% (n = 136) of the cases (P = 0.027) and distant metastasis was more frequent in the young age group (stage M1b was 51.6% vs 28.0%, P = 0.018). Satellite nodule formation in the lung or tumor location was not statistically different between the two groups (Table 2). Significant survival differences were not observed between the young and old age groups with lung adenocarcinoma (P = 0.450) (Fig. 1B).
When evaluating the histologic characteristics of lung adenocarcinoma arising in young patients, a signet ring cell feature was more frequently seen in the young age group, compared with the old age group (25.8% vs 11.5%, P = 0.025). Lepidic growth pattern tended to be less frequently seen in the young age group (3.2% vs 16.5%), although the statistical significance was marginal (P = 0.051). There were no significant differences between the young and old age groups in terms of predominant histologic pattern, tumor differentiation, and presence of a micropapillary feature (Table 3). Adenocarcinoma with a signet ring cell feature showed worse survival rates than adenocarcinoma without a signet ring cell feature in both total adenocarcinoma cohort (P = 0.001) (Fig. 2A) and in young age group (P = 0.018). Patients with adenocarcinoma having a lepidic growth pattern demonstrated better survival rates than those without a lepidic growth pattern only in total adenocarcinoma cohort but not in young age group (P < 0.001 and P = 0.454, respectively) (Fig. 2B). In multivariate analysis, only TNM stage (P < 0.001, HR = 0.111) was the independent prognostic factor among other variables in lung adenocarcinoma cohort. If we excluded the TNM stage in multivariate analysis, only lepidic growth pattern showed independent prognostic significance (P < 0.001, HR = 3.286). However, in young age group, there was no variable having independent prognostic significance.
When the EGFR mutation rate was evaluated using IHC with EGFR mutation specific antibodies, total EGFR mutation positivity was detected in 22.5% (n = 7) and 26.9% (n = 70) of the young and old age groups, respectively. In cases of E746 EGFR mutation, positive staining was observed in 12.9% of the young age group (n = 4) and 12.0% of the old age group (n = 31) (Fig. 3A). Positivity for L858R EGFR mutation was seen in 10.3% of the young age group (n = 3) and 15.4% of the old age group (n = 40) (Fig. 3B). Total EGFR mutational rate of the young age group was not different from that of the old age group (P = 0.606) (Table 3).
ALK was detected in 14 patients (4.8%) (Fig. 3C). Among the young age group, only 1 case showed a positive result (1/31, 3.2%) with a score of (2+). Among the cases in the old age group, 13 patients (5.0%) showed ALK positivity, which included scores of (2+) (n = 7), and (3+) (n = 6). ALK positivity was frequently present in female patients but few in male patients (P = 0.003). There was no statistical difference of ALK positivity between the young and old age groups (P = 0.856) and between smokers and nonsmokers (P = 0.193). ALK-positive adenocarcinoma tended to have a signet ring cell feature, but the statistical significance was marginal (P = 0.068). Survival rate was not statistically different from EGFR mutation positive and negative groups (P = 0.983) and between ALK positive and null groups (P = 0.733).
Lung cancer of young patients is very rare and the percentage of young patients in the previous studies ranged from 1.1% to 5% of total lung cancer patients (1-4). In our study, among the total of 1,255 primary lung carcinoma patients, 52 (4.9%) were aged ≤ 40 yr at the time of diagnosis. When compared with patients in the old age group, females, non-smokers, and those with a more advanced stage at diagnosis and histologic type of adenocarcinoma were predominant in patients with lung cancer in the young age group. This result is consistent with previous studies, which showed a bias toward females, non-smokers, and adenocarcinoma histology (2-7). There were several conflicting results that male (8) and smokers (6) were more prevalent, the severity of disease at presentation was not different (5), and squamous cell carcinoma was more frequently diagnosed than adenocarcinoma (4, 8). Furthermore, several studies have described similar overall survival in young and old patients with lung cancer (3, 6, 7), whereas, others have reported worse (4, 5) or better overall survival (2, 8) in young patients compared with old patients. The discrepancies might be due to the rare occurrence of lung cancer in young patients, differences in study designs enrolling surgically treated cases only or including unresectable cases, and age cut-off levels dividing young and old age groups, and differences in smoking habits, environments and ethnicity in the populations studied. The characteristics of young patients with lung cancer were preserved in those of young patients with adenocarcinoma: a predominance of females, nonsmokers, high TNM stages and high rates of distant metastasis, and similar overall survival. This result is concordant to a previous report showing high percentage of younger patients who were female and were never smoked and similar survival rate (3). Our study demonstrated that overall survival of these two groups was not different in the cohort of total lung cancer and in that of adenocarcinoma, although patients with stage IV lung cancer were predominant in the young age group. More aggressive treatment, better compliance, and fewer coexisting diseases and post-treatment complications might affect the prognosis of young patients with lung cancer (2, 5, 8). Therefore, although the tumor stage is advanced at diagnosis, young patients should be treated more aggressively than the old patient population.
Until now, there have been few comprehensive studies for histologic characteristics of lung adenocarcinoma of young patients. Furthermore, studies about pathologic evaluations of adenocarcinoma did not encompass the cases with advanced stages of the disease (15) although most adenocarcinomas arising in young patients are unresectable at presentation. Therefore, we included surgically resected as well as unresectable advanced lung cancer cases to avoid the bias of patient selection and evaluated the differences of pathologic findings and molecular characteristics of lung adenocarcinoma between the young and old age groups. In cases with advanced lung cancer, we had biopsy slides only and could not evaluate the entire lung cancer tissue in these cases. With this limitation, instead of categorizing adenocarcinoma to the predominant histologic subtype (20), we subdivided the adenocarcinoma according to their predominant histologic patterns. Adenocarcinoma of young age group did not show any difference in terms of predominant histologic patterns. Lung adenocarcinoma with a lepidic predominant type is considered a unique pathologic subtype because of its good prognosis (21) and stepwise progression from atypical adenomatous hyperplasia through adenocarcinoma in situ (22). Raz and Jablons (15) reported that bronchioloalveolar carcinoma was not associated with a young age of occurrence although the patients with bronchioloalveolar carcinoma are more likely women and non-smokers compared with patients with other subtypes of adenocarcinoma. Adenocarcinoma with lepidic predominant pattern in our study does not represent the lepidic predominant adenocarcinoma or adenocarcinoma in situ because we could not evaluate the entire tumor in most cases. Adenocarcinoma with lepidic predominant pattern did not show any statistical difference between young and old age groups. However, we considered as the presence of a lepidic growth pattern when more than 5% of the tumor cells showed their attributes and demonstrated that lepidic growth pattern was rarely seen in lung adenocarcinoma of the young age group over the old age group.
Additionally we showed that a signet ring cell feature was more frequently present in young age group adenocarcinoma. Signet ring cells are a unique cell type identified in mucinous adenocarcinoma. Kish et al. (23) described five cases of primary signet ring cell carcinoma of the lung as a unique subtype of lung adenocarcinoma showing a worse prognosis than adenocarcinoma without signet ring cells. Younger age and non-smoker predominance are the characteristics of lung cancer cases in which the signet ring cell components are predominant (24). Additionally Iwasaki et al. (25) demonstrated that primary lung adenocarcinomas with more than 5% signet ring cell components had a significantly worse survival rate than those with less than 5% signet ring cell components. However, according to new International Association for the Study of Lung Cancer (IASLC) classification of lung adenocarcinoma, signet ring cell carcinoma is removed from the subtypes of adenocarcinoma, because signet ring cell feature is regarded as only cytologic feature that can be associated with various histologic patterns (20). Lung adenocarcinomas with signet ring cells showed worse overall survival than those without signet ring cells in young age group as well as total adenocarcinoma cohort in univariate analysis. Because of a small number of patients in this age group, we failed to demonstrate their significance as independent prognostic factors in multivariate analysis. Although the total incidence of signet ring cell feature was low and its prognostic significance was not confirmed in multivariate analysis, the possibility is suggested that presence of signet ring cells in lung adenocarcinoma might be an indicator of poor survival of lung adenocarcinoma patients in young age and total adenocarcinoma groups. Further study in large cohort is required for evaluation of prognostic significance of the histologic variables in young age groups.
The unique clinicopathologic characteristics of young patients with lung adenocarcinoma support the probability that the cause of young-age adenocarcinoma might be associated with genetic susceptibility or exposure to environmental carcinogens other than active smoking. There are few reports about the genetic changes in young patients with lung adenocarcinoma (26, 27). Gain or high-level amplifications in the long arm of chromosome 20 are observed more frequently and might be important in the tumorigenesis of lung adenocarcinoma of the young age group (26). The proportion of p53-positive cases is significantly lower, but angiogenesis of lung adenocarcinoma is more closely correlated with p53 expression in young patients than in old patients (27). Recently, EGFR mutation and ALK rearrangement are known to be closely related to the responsiveness of specific chemotherapeutic drugs, such as EGFR-TKI (10) and ALK inhibitor (28), as well as be associated with specific demographic features (9, 10, 13, 14). The patients with ALK rearranged lung adenocarcinomas share several clinical characteristics with patients who have adenocarcinoma with a signet ring cell feature. Adenocarcinomas with ALK rearrangement are more likely to present in younger patients without a history of smoking and at a more advanced stage compared with wild type adenocarcinoma (13, 14). Additionally, solid tumor growth and frequent signet-ring cells have been described as a distinct feature of non-small cell lung cancer showing ALK rearrangement (13, 14, 29). The reported percentage of ALK positive NSCLC is 5 to 10% of all lung adenocarcinoma (13, 29). In our study, we identified 14 cases (4.8%) of lung adenocarcinoma with ALK positivity in our cohort. ALK positivity was closely associated with females, but not with young age statistically. ALK-positive adenocarcinoma tended to have signet ring cell features, but showed marginal statistical significance (P = 0.068). These results are somewhat discordant to previous studies and it might be due to small number of patients in young age group and the very rare occurrence of ALK rearrangement. ALK-positive patients were more likely to be younger in most studies (13, 14), but the median age was 51 to 55 yr with an age range of 29 to 76 yr (13, 29). Therefore, when we defined the patients aged 40 yr and younger as the young age group, most patients harboring ALK rearrangement might not to be included in this age group. In addition, the results of IHC for ALK expression do not completely coincide with the FISH results. Paik et al. (14) demonstrated that all FISH-positive patients were assigned ALK IHC scores of (2+) or (3+), all patients with ALK IHC scores of (3+) were FISH-positive, and those with scores of (0) were FISH-negative (14). We deduced that IHC scores of (2+) and (3+) were ALK positive and scores of (0) and (1+) were ALK negative. IHC results for ALK might not be completely in agreement to actual ALK rearrangement status. Therefore, a large-scale analysis with ALK-FISH would be needed to delineate the relationship between ALK rearrangement and young age occurrence of lung adenocarcinoma.
IHC using EGFR mutation-specific antibodies is a rapid, sensitive, and cost-effective method to identify the two most common EGFR mutations in NSCLC tissue samples and could be a screening assay for EGFR mutations especially in small biopsy samples which do not provide enough high quality DNA for direct sequencing (16, 17). Although young patients with lung adenocarcinoma tended to be females and non-smokers, the EGFR mutation rate of them was not different with that of the old patients. Therefore, we supposed that EGFR mutation status might not be associated with the development of young age lung adenocarcinoma.
Because most lung cancers show histologic heterogeneity and about 95% of adenocarcinoma are mixed subtype (12), small biopsy samples are not representative of the entire tumor tissue. A total 68.8% of the cases were small biopsy samples in our study and we could not evaluate histologic features of entire tumor tissues and could not apply the histologic criteria properly to the biopsy samples. If we could have encompassed a large number of cases in the study, we might have overcome this limitation. However, this study included the cases of just one institute and the number of cases of young age adenocarcinoma was not enough to overcome the limitation. Additionally we included metastatic tumor samples in our cohort. The discrepancies between primary tumor and corresponding metastasis exist in about 0%-29% of NSCLC (30, 31). Histologic features and molecular profiles of metastatic tumor might not be representative of those of primary tumor, and highly sensitive detection methods might overcome the discrepancy between primary tumors and metastasis.
Although there are some limitations on this study, we think that this study is unique in terms of its focus on thorough evaluation of histologic characteristics in one specific type of lung cancer (adenocarcinoma), encompassing unresectable as well as surgically treated cases, and on assessment of molecular characteristics such as EGFR mutation status, and ALK rearrangement using IHC in the young patient cohort.
In conclusion, lung adenocarcinoma of young patients demonstrates characteristic clinicopathologic features such as female and non-smoker predominance, high TNM stages, high rates of distant metastasis, the presence of signet ring cell feature, rare occurrence of lepidic growth pattern, and similar overall survival rates. EGFR mutation status might not be associated with the development of young age lung adenocarcinoma. ALK rearrangement in young age lung adenocarcinoma should be evaluated through large-scale analysis, and other genetic abnormalities should be investigated and it would be helpful for the treatment of young patients suffering from lung adenocarcinoma.
Figures and Tables
Fig. 1
Kaplan-Meier Curves comparing overall survival between the young and old age groups. (A) Overall survival in the young and older patients with lung carcinoma is not statistically different (P = 0.893). (B) Overall survival in the young and older patients with lung adenocarcinoma is not statistically different (P = 0.450).

Fig. 2
Kaplan-Meier curves comparing overall survival between presence and absence of signet ring cell feature and between those of lepidic growth pattern. (A) Overall survival of patients having signet ring cell feature is worse than that of patients without signet ring cell feature (P = 0.001). (B) Patients with lepidic growth pattern show better survival rate than those without lepidic growth pattern (P < 0.001).

Fig. 3
Immunohistochemical stains of lung adenocarcinoma cases. (A) and (B) E746 and L858R EGFR mutation-specific antibodies show a positive staining at tumor cells (A, E746 mutation-specific antibody, × 200; B, L858R mutation-specific antibody, × 400). (C) In a case of adenocarcinoma showing signet ring cell feature, ALK positivity is present at tumor cells (ALK, × 400).
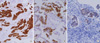
Table 1
Comparison of clinicopathologic findings of the total lung cancer between the young and old age groups

References
1. Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: Incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010. 25:1113–1121.
2. Subramanian J, Morgensztern D, Goodgame B, Baggstrom MQ, Gao F, Piccirillo J, Govindan R. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol. 2010. 5:23–28.
3. Liu NS, Spitz MR, Kemp BL, Cooksley C, Fossella FV, Lee JS, Hong WK, Khuri FR. Adenocarcinoma of the lung in young patients. The M. D. Anderson experience. Cancer. 2000. 88:1837–1841.
4. Green LS, Fortoul TI, Ponciano G, Robles C, Rivero O. Bronchogenic cancer in patients under 40 years old: the experience of a Latin American country. Chest. 1993. 104:1477–1481.
5. Kuo C, Chen Y, Chao J, Tsai C, Perng R. Non-small cell lung cancer in very young and very old patients. Chest. 2000. 117:354–357.
6. Blanco M, Garcia-Fontan E, Rivo JE, Repaaz JR, Obeso GA, Canizares MA. Bronchogenic carcinoma in patients under 50 years old. Clin Transl Oncol. 2009. 11:322–325.
7. Skarin AT, Herbst RS, Leong TL, Bailey A, Sugarbaker D. Lung cancer in patients under age 40. Lung Cancer. 2001. 32:255–264.
8. Tian DL, Liu HX, Zhang L, Yin HN, Hu YX, Zhao HR, Chen DY, Han LB, Li Y, Li HW. Surgery for young patients with lung cancer. Lung Cancer. 2003. 42:215–220.
9. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004. 101:13306–13311.
10. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004. 304:1497–1500.
11. Zakowski MF, Hussain S, Pao W, Ladanyi M, Ginsberg MS, Heelan R, Miller VA, Rusch VW, Kris MG. Morphologic features of adenocarcinoma of the lung predictive of response to the epidermal growth factor receptor kinase inhibitors Erlotinib and Gefitinib. Arch Pathol Lab Med. 2009. 133:470–477.
12. Motoi N, Szoke J, Riely GJ, Seshan VE, Kris MG, Rusch VW, Gerald WL, Travis WD. Lung adenocarcinoma: Modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol. 2008. 32:810–827.
13. Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S, McDermott U, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009. 27:4247–4253.
14. Paik JH, Choe G, Kim H, Choe J, Lee HJ, Lee C, Lee JS, Jheon S, Chung J. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: Correlation with fluorescence in situ hybridization. J Thorac Oncol. 2011. 6:466–472.
15. Raz DJ, Jablons DM. Bronchioloalveolar carcinoma is not associated with younger age at diagnosis: an analysis of the SEER database. J Thorac Oncol. 2006. 1:339–343.
16. Yu J, Kane S, Wu J, Benedettini E, Li D, Reeves C, Innocenti G, Wetzel R, Crosby K, Becker A, et al. Mutation-specific antibodies for the detection of EGFR mutations in non-small-cell lung cancer. Clin Cancer Res. 2009. 15:3023–3028.
17. Brevet M, Arcila M, Ladanyi M. Assessment of EGFR mutation status in lung adenocarcinoma by immunohistochemistry using antibodies specific to the two major forms of mutant EGFR. J Mol Diagn. 2010. 12:169–176.
18. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. Edge SB, editor. Lung. AJCC cancer staging manual. 2010. 7th ed. New York: Springer;253–266.
19. Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. Kleihues P, Sobin LH, editors. Pathology and genetics of tumours of the lung, pleura, thymus and heart. World Health Organization classification of tumours. 2004. Lyon: IARCPress;35–44.
20. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, et al. International Aassociation for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary Classification of lung adenocarcinoma. J Thorac Oncol. 2011. 6:244–285.
21. Noguchi M, Morikawa A, Kawasaki M, Matsuno Y, Yamada T, Hirohashi S, Kondo H, Shimosato Y. Small adenocarcinoma of the lung. Cancer. 1995. 75:2844–2852.
22. Nakano H, Soda H, Takasu M, Tomonaga N, Yamaguchi H, Nakatomi K, Fujino S, Hayashi T, Nakamura Y, Tsukamoto K, et al. Heterogeneity of epidermal growth factor receptor mutations within a mixed adenocarcinoma lung nodule. Lung Cancer. 2008. 60:136–140.
23. Kish JK, Ro JY, Ayala AG, McMurtrey MJ. Primary mucinous adenocarcinoma of the lung with signet-ring cells: a histochemical comparison with signet-ring cell carcinomas of other sites. Hum Pathol. 1989. 20:1097–1102.
24. Tsuta K, Ishii G, Yoh K, Nitadori J, Hasebe T, Nishiwaki Y, Endoh Y, Kodama T, Nagai K, Ochiai A. Primary lung carcinoma with signet-ring cell carcinoma components: clinicopathological analysis of 39 cases. Am J Surg Pathol. 2004. 28:868–874.
25. Iwasaki T, Ohta M, Lefor AT, Kawahara K. Signet-ring cell carcinoma component in primary lung adenocarcinoma: potential prognostic factor. Histopathology. 2008. 52:639–640.
26. Lindstrom I, Nordling S, Nissen A, Tammilehto L, Mattson K, Knuutila S. DNA copy number changes in lung adenocarcinoma in younger patients. Mod Pathol. 2002. 15:372–378.
27. Kondou M, Nagayasu T, Hidaka S, Tsuchiya T, Takeshita H, Yasutake T, Yano H, Minami H, Iwasaki K. Correlation between angiogenesis and p53 expression in lung adenocarcinoma of young patients. Tohoku J Exp Med. 2009. 217:101–107.
28. Shaw AT, Costa DB, Iafrate AJ, Dezube BJ, Shapiro GI, Bang YJ, Janne PA, Lynch TJ, Maki RG, Gamidge DR, et al. Clinical activity of the oral ALK and MET inhibitor PF-02341066 in non-small cell lung cancer (SNCLC) with EML4-ALK translocations. J Thorac Oncol. 2009. 4:S305–S306.
29. Rodig SJ, Mino-Kenudson M, Dacic S, Yeap BY, Shaw A, Barletta JA, Stubbs H, Law K, Lindeman N, Mark E, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009. 15:5216–5223.
30. Jakobsen JN, Sorensen JB. Intratumor heterogeneity and chemotherapy-induced changes in EGFR status in non-small cell lung cancer. Cancer Chemother Pharmacol. 2012. 69:289–299.
31. Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of EGFR mutation is extremely rare in lung adenocarcinoma. J Clin Oncol. 2011. 29:2972–2977.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download