Abstract
We evaluated the correlations between BMI, fasting glucose, insulin, testosterone level, insulin resistance, and prostate size in non-diabetic benign prostatic hyperplasia (BPH) patients with normal testosterone levels. Data from 212 non-diabetic BPH patients with normal testosterone levels, who underwent transurethral resection of the prostate (TURP) due to medical treatment failure, were evaluated retrospectively. Patients with prostate specific antigen (PSA) levels of ≥ 3 ng/mL underwent multicore transrectal prostate biopsy before TURP to rule out prostate cancer. Patients with diabetes mellitus (DM) or serum testosterone levels of < 3.50 ng/mL were excluded from analysis. Correlations between clinical and laboratory parameters were determined. Prostate size correlated positively with age (r = 0.227, P < 0.001), PSA (r = 0.510, P < 0.001), and fasting glucose level (r = 0.186, P = 0.007), but not with BMI, testosterone, insulin level, or insulin resistance (each P > 0.05). Testosterone level inversely correlated with BMI (r = -0.327, P < 0.001), insulin level (r = -0.207, P = 0.003), and insulin resistance (r = -0.221, P = 0.001), but not with age, prostate size, PSA, or fasting glucose level (each P > 0.05). Upon multiple adjusted linear regression analysis, prostate size correlated with elevated PSA (P < 0.001) and increased fasting glucose levels (P = 0.023). In non-DM BPH patients with normal testosterone levels, fasting glucose level is an independent risk factor for prostate hyperplasia.
Benign prostatic hyperplasia (BPH) is the most common prostate disease, characterized by nonmalignant enlargement of the prostate gland in aging men. The prevalence of BPH rises with age (1). Although the exact pathogenesis of BPH is far from completely understood, aging, testosterone levels, clinically significant lower urinary tract symptoms, inflammation, alteration in cell signaling are the significant risk factors for the development of BPH (2, 3). In addition, insulin, insulin-like growth factors (IGFs), diabetes mellitus (DM), and obesity are known androgen-independent risk factors in BPH development (4).
Testosterone is the main circulating androgen in adult males and is secreted primarily by the testis. It also promotes prostate growth by increasing the ratio of cellular proliferation to death. Testosterones and growth factors regulate this ratio by both stimulating proliferation and inhibiting apoptosis (5). Dihydrotestosterone (DHT), a derivative of testosterone, modulates the effects of several growth factors involved in BPH development, including fibroblast growth factors (FGFs), vascular endothelial growth factors (VEGFs), and IGFs (5-8).
In particular, IGFs are regarded as potent inducers of prostate growth in vitro (9, 10), and insulin levels, obesity and DM are considered risk factors for BPH development (11, 12). These factors are associated with a metabolic syndrome characterized by insulin resistance and hyperinsulinemia. Two main components of the metabolic syndrome are obesity and abnormal glucose homeostasis (13). Vikram et al. (14) demonstrated that a high-fat diet resulting in hyperinsulinemia led to increased cellular proliferation and enlargement of the prostate in rats. Also, Nandeesha et al. (15) reported that hyperinsulinemia associated with insulin resistance was an independent risk factor for the development of BPH in non-DM men. Therefore, insulin resistance and hyperinsulinemia play a significant role in the BPH development. However, in non-DM BPH patients with normal testosterone levels, the relationships between body mass index (BMI), insulin level, insulin resistance and prostate growth have not been yet established. This study evaluated these relationships in non-diabetic BPH patients with normal testosterone levels who underwent transurethral resection of the prostate (TURP).
Clinical data from 345 BPH patients who underwent TURP was reviewed retrospectively. From July 1996 to February 2010, patients whose serum was taken on the morning of the operation day among patients underwent TURP were enrolled. To rule out prostate cancer, patients whose prostate specific antigen (PSA) levels were greater than or equal to 3 ng/mL were subjected to multicore transrectal prostate biopsy (generally 12 cores) before TURP. Prostate size was measured by transrectal ultrasound and the volume was calculated from transverse images by using the prolate-ellipsoid formula (0.524 × height × width × length) (16). Patients on medical treatment for DM were excluded, as were patients whose serum testosterone levels were less than 3.50 ng/mL, or who had been placed on any testosterone replacement medications such as testosterone injection or testosterone gel that could affect testosterone levels. Altogether, 212 BPH patients without DM and with normal testosterone levels were included in the analysis.
On the morning of the operation day, patient's serum was taken. Serum PSA levels were measured by using a quantified monoclonal IRMA radioimmunoassay (Izotop, Budapest, Hungary). Glucose levels were measured on a Hitachi 7600 automatic chemical analyzer (Hitachi, Tokyo, Japan) using the hexokinase method. Insulin and testosterone levels were measured by using an Elecsys 2010 autoanalyzer (Roche Diagnostics, Indianapolis, IN, USA) according to electrochemiluminescence immunoassay principles. Insulin levels were measured by a method that employs insulin-specific monoclonal antibodies and is based on the sandwich test principle. The testosterone assay was based on the competitive test principle and employed a polyclonal testosterone-specific antibody. All assays were performed according to the manufacturers' instructions. Insulin resistance was determined by the homeostasis model assessment (HOMA) using the following formula (17):
Correlations between prostate size, testosterone level, insulin resistance, and other clinico-laboratory parameters (age, height, weight, BMI, PSA, fasting glucose, and insulin levels) were assessed. In addition, multiple linear regression analysis was performed to evaluate the association between prostate size and other parameters. Statistical analyses were performed by the Statistical Package for Social Sciences, version 12.0, software (SPSS Inc., Chicago, IL, USA). All tests were performed using a 2-tailed analysis, and a P value of < 0.05 was considered statistically significant.
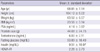
The mean age of the study population was 68.81 ± 7.14 yr and the mean BMI was 23.56 ± 3.14 kg/m2. The mean serum PSA, testosterone, fasting glucose, and insulin levels were 4.14 ± 3.82 ng/mL, 6.00 ± 2.11 ng/mL, 93.89 ± 18.49 mg/dL, and 9.51 ± 10.97 mg/dL, respectively. The mean HOMA-IR and prostate size were 2.26 ± 2.73 and 44.08 ± 24.76 g. Additional baseline characteristics of the patients included in this study are presented in Table 1.
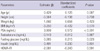
As shown in Table 2, prostate size positively correlated with age (r = 0.227, P < 0.001), PSA (r = 0.510, P < 0.001), and fasting glucose (r = 0.186, P = 0.007), but not with BMI, testosterone, insulin level, or HOMA-IR. Testosterone level inversely correlated with BMI (r = -0.327, P < 0.001), insulin level (r = -0.207, P = 0.003), and HOMA-IR (r = -0.221, P = 0.001), but not with age, prostate size, PSA, or fasting glucose. HOMA-IR significantly correlated with BMI (r = 0.328, P < 0.001), fasting glucose (r = 0.263, P < 0.001), and insulin level (r = 0.975, P < 0.001), but not with age, PSA, or prostate size.
As shown in Table 3, in multiple adjusted linear regression analysis, prostate size was significantly associated with PSA (P < 0.001) and fasting glucose level (P = 0.023). However, prostate size was not related to age, BMI, testosterone, insulin level, or HOMA-IR.
In this study, several well-known risk factors for the development of BPH were evaluated in BPH patients with normal testosterone levels and no evidence of DM. Fasting glucose level was the only independent risk factor for prostatic hyperplasia in these patients. Other factors, including obesity, hyperinsulinemia, and insulin resistance, were not significantly associated with prostate size.
Many previous studies have demonstrated that obesity, DM, high insulin, and low HDL cholesterol are risk factors for the development of BPH (12, 14, 15). It is likely that obesity promotes BPH by inducing systemic inflammation and oxidative stress (18). Inflammatory mediators and oxidative stress could possibly promote unregulated prostate growth through a nonmalignant pathway (19, 20). It is also possible that alterations in the balance between testosterone and estrogen levels in prostate tissue contribute to BPH development (21), because increased adipose tissue promotes increased aromatization of circulating testosterone into estrogen (22). However, in the current study of non-DM BPH patients with normal testosterone levels, BMI was not correlated with prostate size. In our previous study (23), the positive correlation between BMI and prostate size in pathologically proven BPH patients might be due to the inclusion of patients with DM and low testosterone levels. These findings suggest that obesity may not be an independent risk factor for the development of BPH in non-DM patients with normal testosterone levels.
Insulin resistance, a condition in which a normal level of insulin elicits a subnormal response, is associated with a group of disorders such as obesity, dyslipidemia, elevated fasting glucose levels, hyperinsulinemia, and hypertension (4). Recent experimental animal studies showed that hyperinsulinemia led to increased cellular proliferation and enlargement of the prostate in rats (14). In addition, several studies have shown that insulin-resistance and hyperinsulinemia could be independent risk factors for BPH (15, 24). In the present study, there was no correlation between insulin level, or insulin resistance and prostate size. Vikram et al. (4) suggested that prostate growth by hyperinsulinemia could be attributed to the 1) enhanced mitogenic activity of insulin; 2) altered steroidal hormonal activity; 3) increased sympathetic tone; or 4) perturbed endocrine levels in the prostate. However, in the current study of non-DM BPH patients with normal testosterone levels, no relationship between prostate growth and the above-mentioned metabolic factors was observed.
Although normal prostate growth is known to be testosterone-dependent, higher testosterone levels do not necessarily result in enhanced prostate growth (25). For example, in hypogonadal men, testosterone replacement increased PSA and prostate size, mainly during the first 6 months of therapy, to levels equivalent to those of men without hypogonadism but no higher (26). Furthermore, within the range of normal testosterone levels, there is no clear relationship between the concentration of circulating testosterone and prostate size in aging males (27). In this study, there was no correlation between testosterone level and prostate size in non-DM BPH patients with normal testosterone levels.
Low testosterone levels may predispose males to visceral obesity, which leads to the dysregulation of fatty acid metabolism, which in turn promotes insulin resistance (28). Testosterone inhibits lipoprotein lipase activity in abdominal adipose tissue, which leads to decreased triglyceride uptake in central fat depots (29). Pitteloud et al. (30) reported that low serum testosterone levels were associated with an adverse metabolic profile and that low testosterone levels promoted insulin resistance in men. They also reported that serum testosterone levels were positively related to insulin sensitivity (30); nevertheless, it does not necessarily follow that serum testosterone levels are inversely associated with insulin resistance. In the current study, serum testosterone levels were inversely correlated with BMI, insulin level, and HOMA-IR. In addition, because low testosterone levels are a consequence of the dysregulation of fatty acid metabolism and BPH patients with low testosterone were excluded, patients with insulin resistance due to the dysregulation of fatty acid metabolism were not evaluated within this study. Thus, metabolic dysregulation does not contribute to prostate growth in non-DM BPH patients with normal testosterone levels.
In the present study, multiple regression analysis revealed that serum fasting glucose levels correlated positively with prostate size. Parsons et al. (12) reported that obesity, elevated fasting glucose and diabetes were risk factors for BPH. In their study of 422 men, obesity and DM as well as fasting glucose were related to prostate size. However, that study included patients with diabetes and/or low testosterone levels. Altogether, these findings suggest that other unknown factors may influence prostate growth in non-DM patients with normal testosterone levels through mechanisms other than testosterone, obesity, or abnormal glucose homeostasis. Furthermore, lowering fasting glucose levels by drug and exercise might decrease prostate size and reduce the need for TURP in these patients.
In conclusion, this study is the first to examine the relationships between BMI, insulin levels, insulin resistance, and prostate size in non-DM BPH patients with normal testosterone levels. In non-DM BPH patients with normal testosterone levels, prostate size correlated with fasting glucose levels but not with BMI, testosterone levels, insulin levels, or insulin resistance. Therefore, in these patients, fasting glucose level is an independent risk factor for prostate hyperplasia. Consequently, further studies examining the relationship between BMI, insulin resistance and prostate growth in different races are warranted, along with prospective, population-based studies in healthy individuals to evaluate the relationship between fasting blood glucose levels and the risk of developing BPH.
Figures and Tables
AUTHOR SUMMARY
Prostate Size Correlates with Fasting Blood Glucose in Non-Diabetic Benign Prostatic Hyperplasia Patients with Normal Testosterone Levels
Won Tae Kim, Seok Joong Yun, Young Deuk Choi, Gi-Young Kim, Sung-Kwon Moon, Yung Hyun Choi, Isaac Yi Kim and Wun-Jae Kim
Recently, hyperinsulinemia associated with insulin resistance has also been shown to be an independent risk factor for BPH. We evaluated the correlations between BMI, fasting glucose, insulin, testosterone level, insulin resistance, and prostate size in 212 non-diabetic BPH patients with normal testosterone levels. In these patients, prostate size correlated with fasting glucose levels but not with BMI, testosterone level, insulin, and insulin resistance.
References
1. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984. 132:474–479.
2. Lee C, Kozlowski JM, Grayhack JT. Etiology of benign prostatic hyperplasia. Urol Clin North Am. 1995. 22:237–246.
3. Donnell RF. Benign prostate hyperplasia: a review of the year's progress from bench to clinic. Curr Opin Urol. 2011. 21:22–26.
4. Vikram A, Jena G, Ramarao P. Insulin-resistance and benign prostatic hyperplasia: the connection. Eur J Pharmacol. 2010. 641:75–81.
5. Lee KL, Peehl DM. Molecular and cellular pathogenesis of benign prostatic hyperplasia. J Urol. 2004. 172:1784–1791.
6. Story MT, Livingston B, Baeten L, Swartz SJ, Jacobs SC, Begun FP, Lawson RK. Cultured human prostate-derived fibroblasts produce a factor that stimulates their growth with properties indistinguishable from basic fibroblast growth factor. Prostate. 1989. 15:355–365.
7. Walsh K, Sriprasad S, Hopster D, Codd J, Mulvin D. Distribution of vascular endothelial growth factor (VEGF) in prostate disease. Prostate Cancer Prostatic Dis. 2002. 5:119–122.
8. Peehl DM, Cohen P, Rosenfeld RG. The insulin-like growth factor system in the prostate. World J Urol. 1995. 13:306–311.
9. Cohen P, Peehl DM, Lamson G, Rosenfeld RG. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins in primary cultures of prostate epithelial cells. J Clin Endocrinol Metab. 1991. 73:401–407.
10. Monti S, Di Silverio F, Lanzara S, Varasano P, Martini C, Tosti-Croce C, Sciarra F. Insulin-like growth factor-I and -II in human benign prostatic hyperplasia: relationship with binding proteins 2 and 3 and androgens. Steroids. 1998. 63:362–366.
11. Dahle SE, Chokkalingam AP, Gao YT, Deng J, Stanczyk FZ, Hsing AW. Body size and serum levels of insulin and leptin in relation to the risk of benign prostatic hyperplasia. J Urol. 2002. 168:599–604.
12. Parsons JK, Carter HB, Partin AW, Windham BG, Metter EJ, Ferrucci L, Landis P, Platz EA. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006. 91:2562–2568.
13. Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome. Circulation. 2003. 108:1541–1545.
14. Vikram A, Jena GB, Ramarao P. Increased cell proliferation and contractility of prostate in insulin resistant rats: linking hyperinsulinemia with benign prostate hyperplasia. Prostate. 2010. 70:79–89.
15. Nandeesha H, Koner BC, Dorairajan LN, Sen SK. Hyperinsulinemia and dyslipidemia in non-diabetic benign prostatic hyperplasia. Clin Chim Acta. 2006. 370:89–93.
16. Matthews GJ, Motta J, Fracehia JA. The accuracy of transrectal ultrasound prostate volume estimation: clinical correlations. J Clin Ultrasound. 1996. 24:501–505.
17. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28:412–419.
18. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004. 114:1752–1761.
19. Wang W, Bergh A, Damber JE. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate. 2004. 61:60–72.
20. Suzuki S, Platz EA, Kawachi I, Willett WC, Giovannucci E. Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia. Am J Clin Nutr. 2002. 75:689–697.
21. Shibata Y, Ito K, Suzuki K, Nakano K, Fukabori Y, Suzuki R, Kawabe Y, Honma S, Yamanaka H. Changes in the endocrine environment of the human prostate transition zone with aging: simultaneous quantitative analysis of prostatic sex steroids and comparison with human prostatic histological composition. Prostate. 2000. 42:45–55.
22. Hautanen A. Synthesis and regulation of sex hormone-binding globulin in obesity. Int J Obes Relat Metab Disord. 2000. 24:Suppl 2. S64–S70.
23. Kim WT, Choi YD, Park C, Kim YW, Yun SJ, Kim IY, Kim WJ. Parathyroid hormone is not involved in prostate growth in patients with benign prostatic hyperplasia. Prostate. 2011. 71:1210–1215.
24. Hammarsten J, Högstedt B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol. 2001. 39:151–158.
25. Morgentaler A. Testosterone replacement therapy and prostate risks: where's the beef? Can J Urol. 2006. 13:Suppl 1. 40–43.
26. Meikle AW, Arver S, Dobs AS, Adolfsson J, Sanders SW, Middleton RG, Stephenson RA, Hoover DR, Rajaram L, Mazer NA. Prostate size in hypogonadal men treated with a nonscrotal permeation-enhanced testosterone transdermal system. Urology. 1997. 49:191–196.
27. Tan MO, Karabiyik I, Uygur MC, Diker Y, Erol D. Serum concentrations of sex hormones in men with severe lower urinary tract symptoms and benign prostatic hyperplasia. Int Urol Nephrol. 2003. 35:357–363.
28. Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, Coleman E, Smith C. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest. 1991. 88:960–966.
29. Mårin P, Odén B, Björntorp P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab. 1995. 80:239–243.
30. Pitteloud N, Mootha VK, Dwyer AA, Hardin M, Lee H, Eriksson KF, Tripathy D, Yialamas M, Groop L, Elahi D, Hayes FJ. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005. 28:1636–1642.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



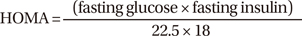

 XML Download
XML Download