Abstract
Although the incidence of bleeding complications during extracorporeal membrane oxygenator (ECMO) support has decreased in various trials, bleeding is still the most fatal complication. We investigated the ideal dosage and efficacy of nafamostat mesilate for use with ECMO in patients with acute cardiac or respiratory failure. We assessed 73 consecutive patients who received ECMO due to acute cardiac or respiratory failure between January 2006 and December 2009. To evaluate the efficacy of nafamostat mesilate, we divided the patients into 2 groups according to the anticoagulants used during ECMO support. All patients of nafamostat mesilate group were male with a mean age of 49.2 yr. Six, 3, 5, and 3 patients were diagnosed with acute myocardial infarction, cardiac arrest, septic shock, and acute respiratory distress syndrome, respectively. The mean dosage of nafamostat mesilate was 0.64 mg/kg/hr, and the mean duration of ECMO was 270.7 hr. The daily volume of transfused packed red blood cells, fresh frozen plasma, and cryoprecipitate and the number of complications related to hemorrhage and thrombosis was lower in the nafamostat mesilate group than in the heparin group. Nafamostat mesilate should be considered as an alternative anticoagulant to heparin to reduce bleeding complications during ECMO.
When the treatment of acute heart failure and respiratory failure is difficult with existing conventional methods, treatment with an extracorporeal membrane oxygenator (ECMO) has become an alternative (1). Although heparin has been used as an anticoagulant among a variety of methods to reduce bleeding complications during ECMO usage, bleeding complications still occur in 10%-30% of patients (1-4).
Nafamostat mesilate, a synthetic protease inhibitor with a short half life, has been widely used as an anticoagulant for hemodialysis patients with a tendency to bleed (5-7). It has been reported that nafamostat mesilate produces good results when it is applied for anticoagulation to cardiac surgery and ECMO; however, different dosages were used in each study (8-12).
The present study investigated the ideal dosage and efficacy of nafamostat mesilate for use with ECMO in patients with acute cardiac or respiratory failure.
We retrospectively reviewed the records of 73 consecutive patients who received ECMO due to acute cardiac or respiratory failure between January 2006 and December 2009. Nafamostat mesilate (SK Chemicals Life Science Biz., Seoul, Korea; licensed by Torii Pharmaceutical Co., Ltd., Tokyo, Japan) was used as an anticoagulant in 17 patients and 56 patients received heparin. We excluded 5 of the 56 heparin patients because they underwent an operation just after ECMO: 3 pulmonary artery thromboembolectomies due to acute pulmonary thromboembolism, 1 coronary artery bypass graft due to acute myocardial infarction, and 1 reoperation due to cardiac arrest at 10 hr after receiving a coronary artery bypass graft. We started to use nafamostat mesilate in October 2008 as an anticoagulant during ECMO support in patients with acute renal failure or with a high risk of bleeding due to antiplatelet medication.
The criteria for venoarterial ECMO use was refractory cardiogenic shock, cardiac arrest, and septic shock that did not respond to conventional therapy. The inclusion criteria were a systolic blood pressure < 80 mmHg despite adequate intravascular volume replacement and the infusion of high dose catecholamines (dopamine > 30 µg/kg/min and/or norepinephrine > 0.2 µg/kg/min). The inclusion criteria for venovenous ECMO were based on the lung dysfunction measured with a PaO2/FiO2 ratio < 100 for an FiO2 of 1.0, or an arterial blood gas pH < 7.25 due to hypercapnia despite receiving the optimal treatment.
Three types of centrifugal pumps were used for the ECMO system: Capiox Emergency Bypass System® (Terumo, Inc., Tokyo, Japan), Bio-pump® (Medtronic Inc, Minneapolis, MN, USA), and Centrifugal Rotaflow pump® (Maquet Inc., Hirrlingen, Germany). We used 3 types of polypropylene hollow-fiber membrane oxygenators: CAPIOX® RX25 (Termo, Inc.), D903 AVANT (Sorin Group Italia S.R.L., Mirandola Modena, Italy), and Capiox Emergency Bypass System® (Terumo, Inc.). To enable ECMO, all patients received 17-21 Fr arterial cannulae (DLP®: Medtronic Inc., or RMI®: Edward's Lifescience LLC, Irvine, CA, USA) and 17-28 Fr venous cannulae (DLP®: Medtronic Inc. or RMI®: Edward's Lifescience LLC) according to the patient's size. ECMO was administered in the cardiac catheterization laboratory, except for 3 patients. After the intravenous injection of 10 mg/kg heparin, the femoral artery and femoral vein were cannulated percutaneously using the Seldinger method, except for 1 patient who was cannulated after an inguinal incision. In the case of venovenous ECMO, both femoral veins were cannulated.
Initially, 3 patients were observed for a high infusion rate of nafamostat mesilate (1.15-2.19 mg/kg/hr) to maintain the activated clotting time (ACT) at 140-180 sec. From the 4th patient, nafamostat mesilate was adjusted at 0.41-0.93 mg/kg/hr according to the activated partial thromboplastin time (aPTT; target range: 60-80 sec). The heparin patients received a continuous infusion at a rate of 1-2 mg/kg/hr to maintain the ACT at 140-180 sec. The patients with acute myocardial infarction (AMI) who received percutaneous coronary intervention were administered aspirin (250 mg) and clopidogrel (300 mg) on the day of the procedure, and received aspirin (200 mg) and clopidogrel (75 mg) on the following day.
We tried to maintain the hematocrit levels > 35% and the platelet count > 80,000 per µL. If the hematocrit and platelet counts fell below these levels, we transfused blood products. In addition, fresh frozen plasma (FFP) and cryoprecipitate transfusion was performed once after 3 or 4 days according to the occurrence of hemorrhagic complications.
After weaning was successfully performed, the cannulae were surgically removed in the operating room to avoid complications such as leg ischemia, aneurysm, or arteriovenous fistula. Nafamostat mesilate was discontinued after removing the cannulae, while heparin was discontinued at 12 hr before the cannulae were removed.
The DBSTAT program (ver. 4.1; DBSTAT Co., Chuncheon, Korea) was used for all statistical analysis. Categorical variables were expressed as percentages and evaluated with Pearson's chi-square test or Fisher's exact test. Continuous variables were expressed as the mean and standard deviation and were evaluated with Student's t test or the Mann Whitney U-test. All P values were 2 sided, and P < 0.05 was considered significant.
The clinical characteristics of the 17 patients treated with nafamostat mesilate during ECMO support are as follows. All patients were male, and their mean age was 49.2 yr. Six, 3, 5, and 3 patients were diagnosed with AMI, cardiac arrest, septic shock, and acute respiratory distress syndrome (ARDS), respectively. ECMO in the venoarterial mode was applied to 14 patients. The mean dosage of nafamostat mesilate was 0.64 mg/kg/hr to maintain an aPTT of 60-80 sec. The mean duration of ECMO was 270.7 hr. Seven patients survived and were discharged.
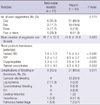
To evaluate the efficacy of nafamostat mesilate, the patients were divided into 2 groups according to the anticoagulants used during ECMO. A clinical comparison between the nafamostat mesilate and heparin groups is shown in Table 1. The patients' ages, sex, diagnosis, ECMO duration, and peak blood urea nitrogen, and total and direct bilirubin levels were found to be statistically significant variables (P < 0.05). The number of mortalities within 24 hr after applying ECMO in the heparin group was 14, which was 1 in the nafamostat mesilate group; however, this was not a significant difference (P = 0.092).
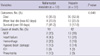
The differences between hemorrhagic and thrombotic complications are shown in Table 2. The average time of oxygenator use in the nafamostat mesilate group was longer than in the heparin group (P = 0.003). The daily transfusion volume of packed RBCs, FFP, and cryoprecipitate was lower in the nafamostat mesilate group than in the heparin group (P < 0.001, P < 0.001, P = 0.035). The number of complications related to hemorrhage and thrombosis was higher in the heparin group (34 in 31 patients) than in the nafamostat mesilate group (4 complications in 4 patients) (P = 0.011). The long term survival and cause of death according to anticoagulants is shown in Table 3. The ECMO support of 8 patients in the heparin group was withdrawn because of uncontrolled bleeding. The nafamostat mesilate group provided a better survival rate than the heparin group (P = 0.048). Sixteen survivors were discharged from the hospital and found still alive in December 2010.
The number of cases in which ECMO is used to treat cardiopulmonary failure is increasing. Although the incidence of bleeding complications has decreased in various trials, bleeding remains one of the most fatal complications. Nafamostat mesilate is a synthetic serine protease inhibitor with a very short half-life that inhibits coagulation, fibrinolysis, and platelet aggregation by inactivating the action of thrombin, activated coagulation factors XIIa and Xa, complement C1r and C1s, plasmin, trypsin, and kallikrein (13-15). Nafamostat mesilate has been used as an anticoagulant instead of heparin for extracorporeal oxygenation, left ventricular assist devices, cardiac surgery, and hemodialysis in patients at high risk of hemorrhage (5, 8, 9, 16).
The anticoagulant effect of nafamostat mesilate, like heparin, is dependent on the dosage; however, the ideal dosage of nafamostat mesilate for ECMO has not yet been determined. According to 2 case studies that administered nafamostat mesilate instead of heparin as an anticoagulant to treat pulmonary hemorrhage during ECMO use, Daimon et al. (10) and Kotani et al. (11) used a nafamostat mesilate infusion rate of 1.0 mg/kg/hr and 1.0-1.7 mg/kg/hr, and ACT was maintained at 150 sec in a 49-yr-old male patient and 190-200 sec in a 27-yr-old female patient, respectively. Nagaya et al. (8) successfully treated bleeding complications in 12 neonates on ECMO by administering nafamostat mesilate at 0.48 mg/kg/hr and heparin at 21.0 U/kg/hr, while ACT was maintained at 205.7 sec. In the present study, a nafamostat mesilate infusion rate of 1.15-2.19 mg/kg/hr was needed to maintain an ACT of 140-180 sec in 3 patients; however, in the other patients, the infusion rate of nafamostat mesilate was lower (0.64 mg/kg/hr), while maintaining an aPTT of 60-80 sec. The test results of ACT are affected by patient characteristics and technical factors (17-19). In this study, the use of aPTT monitoring in the nafamostat mesilate group might allow more precise control of anticoagulation than ACT monitoring. Thrombotic complications were not found at the lower dosage. Two cases of amputation due to leg ischemia occurred in patients who were diagnosed with septic shock; however, these were related to the existing disease, disseminated intravascular coagulation, and not to the insertion of the cannula. A patient who was diagnosed with AMI and acute aortic dissection died after 24 hr due to left hemothorax. Pulmonary hemorrhagic complications occurred in another patient who was diagnosed with septic shock, and he was relieved by nafamostat mesilate; however, the patient died after 404 hr on ECMO because of a previously existing disease. The incidence of bleeding complications in the nafamostat mesilate group was less than in the heparin group.
Transfusion with packed RBCs is needed because hemoglobin levels are decreased during ECMO due to thrombocytopenia, hemolysis, and hemodilution caused by continuous systemic heparinization (20). Formica et al. (21) reported that a transfusion volume of 3.1 units/day was required. Bakhtiary et al. (22) transfused 16.3 units of packed RBCs over an average of 6.3 day during ECMO. Patients with post-cardiotomy and non-cardiotomy received transfusions of 23.3 and 17.3 units of packed RBCs over an average of 5.5 and 11.6 day on ECMO, respectively (23). In the present study, the incidence of bleeding complications in the nafamostat mesilate group was significantly less than in the heparin group because 1.6 units/day of packed RBCs were transfused in the nafamostat mesilate group compared to 7.5 units/day in the heparin group. Such a large transfusion volume was needed in the heparin group because bleeding complications occurred in 31 patients, and, in particular, the greatest transfusion volume was used in the 13 patients who died from bleeding.
We had to change the circuit every 2-3 days due to failure of the oxygenator because we do not have access to long-term support ECMO oxygenators. The average time on ECMO oxygenators in the nafamostat mesilate group was 91.7 hr, which was longer than in the heparin group (41.6 hr) because 14 patients (27.5%) in the heparin group died within 24 hr. This was similar to the report of Sung et al. (24), in which short-term support ECMO was replaced once by the use of an oxygenator for 2-3 days. The duration of ECMO for the nafamostat mesilate group was also longer than for the heparin group because 47% of the nafamostat mesilate patients underwent ECMO due to septic shock or ARDS, and 73% of the heparin patients underwent ECMO due to AMI. Therefore, the different existing diseases had differing recovery periods, resulting in the varying durations of ECMO (20, 25-27).
The recent animal study of Schwertz et al. (28) reported that complement and neutrophils were suppressed by nafamostat mesilate before reperfusion; therefore, myocardial damage was decreased after reperfusion. The survival rate of the AMI patients who were treated with nafamostat mesilate was 50% (3 of 6 patients), which was higher than in the patients who were treated with heparin (11%, 4 of 37 patients). However, additional studies are needed because the small number of patients is not sufficient to determine whether the survival rate is increased due to decreased myocardial damage by nafamostat mesilate.
Results obtained in this study have several limitations. First, our data describe a retrospective analysis of our experience with mixed indication for ECMO implantation. Therefore it is difficult to exclude that higher prevalence of AMI and elderly in the heparin group influenced our results. Second, this study is limited in its ability to suggest the ideal dosage of nafamostat mesilate because it is a retrospective examination of a small number of patients who underwent ECMO for various diagnoses. However, in the present study, the incidence of bleeding complications and transfusions in patients on ECMO decreased when a lower dosage of nafamostat mesilate was needed maintain the aPTT than for the dosages used in previous studies to maintain the ACT. Higher amounts of peak total and direct bilirubin were observed in the nafamostat mesilate patients than in the heparin patients; however, additional studies are needed to examine whether this was caused by the long-term administration of nafamostat mesilate or existing diseases.
In conclusion, nafamostat mesilate should be considered as an alternative anticoagulant to heparin to reduce bleeding complications during ECMO.
Figures and Tables
Table 1
Patients' clinical characteristics according to anticoagulants

SD, standard deviation; BMI, body mass index; AMI, acute myocardial infarction; ARDS, adult respiratory stress syndrome; ECMO, extracorporeal membrane oxygenation; CPR, cardiopulmonary resustitation; VA, venoarterial; VV, venovenous; SOFA, Sepsis-related Organ Failure Assessment; SAPS, Simplified Acute Physiologic Score; i, initial; p, peak; BUN, blood urea nitrogen; Cr, creatinine; TB, total bilirubin; DB, direct bilirubin; CRRT, continuous renal replacement therapy.
AUTHOR SUMMARY
Use of Nafamostat Mesilate as an Anticoagulant during Extracorporeal Membrane Oxygenation
Sang Jin Han, Hyoung Soo Kim, Gun Il Kim, Sung Mi Whang, Kyung Soon Hong, Won Ki Lee and Sun Hee Lee
We investigated the effective dosage and efficacy of nafamostat mesilate for use with extracorporeal membrane oxygenator (ECMO) in patients with acute cardiac or respiratory failure. The daily volume of transfused packed red blood cells, fresh frozen plasma, and cryoprecipitate was lower in patients treated with nafamostat mesilate than with heparin. Also, the number of complications related to hemorrhage and thrombosis was lower than the heparin group. Nafamostat mesilate could be considered as an alternative anticoagulant to heparin during ECMO.
References
1. Bartlett RH, Gattinoni L. Current status of extracorporeal life support (ECMO) for cardiopulmonary failure. Minerva Anestesiol. 2010. 76:534–540.
2. von Segesser LK, Turina M. Cardiopulmonary bypass without systemic heparinization. Performance of heparin-coated oxygenators in comparison with classic membrane and bubble oxygenators. J Thorac Cardiovasc Surg. 1989. 98:386–396.
3. Teng CL, Kim JS, Port FK, Wakefield TW, Till GO, Yang VC. A protamine filter for extracorporeal blood heparin removal. ASAIO Trans. 1988. 34:743–746.
4. Hursting MJ, Dubb J, Verme-Gibboney CN. Argatroban anticoagulation in pediatric patients: a literature analysis. J Pediatr Hematol Oncol. 2006. 28:4–10.
5. Ohtake Y, Hirasawa H, Sugai T, Oda S, Shiga H, Matsuda K, Kitamura N. Nafamostat mesylate as anticoagulant in continuous hemofiltration and continuous hemodiafiltration. Contrib Nephrol. 1991. 93:215–217.
6. Akizawa T, Koshikawa S, Ota K, Kazama M, Mimura N, Hirasawa Y. Nafamostat mesilate: a regional anticoagulant for hemodialysis in patients at high risk for bleeding. Nephron. 1993. 64:376–381.
7. Yang JW, Han BG, Kim BR, Lee YH, Kim YS, Yu JM, Choi SO. Superior outcome of nafamostat mesilate as an anticoagulant in patients undergoing maintenance hemodialysis with intracerebral hemorrhage. Ren Fail. 2009. 31:668–675.
8. Nagaya M, Futamura M, Kato J, Niimi N, Fukuta S. Application of a new anticoagulant (Nafamostat Mesilate) to control hemorrhagic complications during extracorporeal membrane oxygenation--a preliminary report. J Pediatr Surg. 1997. 32:531–535.
9. Ota T, Okada K, Kano H, Okita Y. Cardiopulmonary bypass using nafamostat mesilate for patients with infective endocarditis and recent intracranial hemorrhage. Interact Cardiovasc Thorac Surg. 2007. 6:270–273.
10. Daimon S, Umeda T, Michishita I, Wakasugi H, Genda A, Koni I. Goodpasture's-like syndrome and effect of extracorporeal membrane oxygenator support. Intern Med. 1994. 33:569–573.
11. Kotani K, Ichiba S, Andou M, Sano Y, Date H, Tedoriya T, Goto K, Shimizu N. Extracorporeal membrane oxygenation with nafamostat mesilate as an anticoagulant for massive pulmonary hemorrhage after living-donor lobar lung transplantation. J Thorac Cardiovasc Surg. 2002. 124:626–627.
12. Hong WK, Kim GW, Lee SH, Lee WJ, Yoon DH, Kim HS, Han SJ. A case involving the use of nafamostat mesilate as an anticoagulant during extracorporeal membrane oxygenation in acute myocardial infarction. Korean J Med. 2010. 79:181–186.
13. Hitomi Y, Ikari N, Fujii S. Inhibitory effect of a new synthetic protease inhibitor (FUT-175) on the coagulation system. Haemostasis. 1985. 15:164–168.
14. Fuse I, Higuchi W, Toba K, Aizawa Y. Inhibitory mechanism of human platelet aggregation by nafamostat mesilate. Platelets. 1999. 10:212–218.
15. Fujii S, Hitomi Y. New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin. Biochim Biophys Acta. 1981. 661:342–345.
16. Takahama T, Kanai F, Hiraishi M, Onishi K, Yamazaki Z, Furuse A, Asano K, Yoshitake T, Kazama M. Application of a prostacyclin analogue (PG) and protease inhibitor (FUT) as anticoagulants with an LVAD system. ASAIO Trans. 1986. 32:253–257.
17. Martindale SJ, Shayevitz JR, D'Errico C. The activated coagulation time: suitability for monitoring heparin effect and neutralization during pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 1996. 10:458–463.
18. Baird CW, Zurakowski D, Robinson B, Gandhi S, Burdis-Koch L, Tamblyn J, Munoz R, Fortich K, Pigula FA. Anticoagulation and pediatric extracorporeal membrane oxygenation: impact of activated clotting time and heparin dose on survival. Ann Thorac Surg. 2007. 83:912–919.
19. Oliver WC. Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth. 2009. 13:154–175.
20. Ang AL, Teo D, Lim CH, Leou KK, Tien SL, Koh MB. Blood transfusion requirements and independent predictors of increased transfusion requirements among adult patients on extracorporeal membrane oxygenation - a single centre experience. Vox Sang. 2009. 96:34–43.
21. Formica F, Avalli L, Colagrande L, Ferro O, Greco G, Maggioni E, Paolini G. Extracorporeal membrane oxygenation to support adult patients with cardiac failure: predictive factors of 30-day mortality. Interact Cardiovasc Thorac Surg. 2010. 10:721–726.
22. Bakhtiary F, Keller H, Dogan S, Dzemali O, Oezaslan F, Meininger D, Ackermann H, Zwissler B, Kleine P, Moritz A. Venoarterial extracorporeal membrane oxygenation for treatment of cardiogenic shock: clinical experiences in 45 adult patients. J Thorac Cardiovasc Surg. 2008. 135:382–388.
23. Liden H, Wiklund L, Haraldsson A, Berglin E, Hultman J, Dellgren G. Temporary circulatory support with extra corporeal membrane oxygenation in adults with refractory cardiogenic shock. Scand Cardiovasc J. 2009. 43:226–232.
24. Sung K, Lee YT, Park PW, Park KH, Jun TG, Yang JH, Ha YK. Improved survival after cardiac arrest using emergent autopriming percutaneous cardiopulmonary support. Ann Thorac Surg. 2006. 82:651–656.
25. Nehra D, Goldstein AM, Doody DP, Ryan DP, Chang Y, Masiakos PT. Extracorporeal membrane oxygenation for nonneonatal acute respiratory failure: the Massachusetts General Hospital experience from 1990 to 2008. Arch Surg. 2009. 144:427–432.
26. Combes A, Leprince P, Luyt CE, Bonnet N, Trouillet JL, Léger P, Pavie A, Chastre J. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008. 36:1404–1411.
27. Chen JS, Ko WJ, Yu HY, Lai LP, Huang SC, Chi NH, Tsai CH, Wang SS, Lin FY, Chen YS. Analysis of the outcome for patients experiencing myocardial infarction and cardiopulmonary resuscitation refractory to conventional therapies necessitating extracorporeal life support rescue. Crit Care Med. 2006. 34:950–957.
28. Schwertz H, Carter JM, Russ M, Schubert S, Schlitt A, Buerke U, Schmidt M, Hillen H, Werdan K, Buerke M. Serine protease inhibitor nafamostat given before reperfusion reduces inflammatory myocardial injury by complement and neutrophil inhibition. J Cardiovasc Pharmacol. 2008. 52:151–160.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download