Abstract
Benzo(a)pyrene (BaP) is a polycyclic aromatic hydrocarbon (PAH) that is easily introduced to humans via consumption of grilled or smoked meat. BaP causes harmful oxidative effects on cell development, growth and survival through an increase in membrane lipid peroxidation, oxidative DNA damage and mutagenesis. Therefore, the present study was conducted to evaluate the synergistic effects of BaP on oxidative stress in hepatic tumors. In this study, we established a hepatic tumor model by injecting rat hepatoma N1-S1 cells into healthy rats. Changes in the abundance of heat shock proteins (HSPs), antioxidant enzymes and pro-inflammatory cytokines were then investigated by western blot analysis. In addition, we examined changes in oxidative stress levels. Injection of N1-S1 cells or concomitant injection of BaP and N1-S1 cells resulted in the formation of hepatic tumors at the injection site. Evaluation of rat plasma reveals that hepatic tumors induced by BaP and N1-S1 cells expresses higher levels of Hsp27, superoxide dismutase (SOD), and tumor necrosis factor-α (TNF-α) when compared to those induced by N1-S1 cells only. The collective results of this study suggest that BaP exerts synergistic effects on the expression of HSP, cytokines and antioxidant enzymes in hepatic tumors induced by rat hepatoma N1-S1 cells.
Polycyclic aromatic hydrocarbons (PAHs) are common pollutants found in our daily living environments. Incomplete combustion of organic materials, such as residential heating, refuse incineration and vehicle exhausts are the major sources of PAH production. The International Agency for Research on Cancer (IARC) has characterized PAHs as carcinogens. IARC also classified Benzo(a)pyrene (BaP) as a Group 2A chemical by carcinogenicity (1). Metabolic activation of BaP by cytochrome P450 enzymes leads to formation of the reactive metabolite, BaP-7,8-diol-9,10-epoxide (BPDE). This reactive metabolite has mutagenic and carcinogenic effects that result from its ability to react with DNA to form miscoding adducts, which leads to the initiation of carcinogenesis (2).
In addition, oxidative damage induced by BaP also plays an important role in its carcinogenesis. BaP easily generates reactive oxygen species, which in turn produce harmful oxidative effects against cell development, growth and survival via an increase in membrane lipid peroxidation, oxidative DNA damage and mutagenesis (3). Further, hydroxylated radical cationic forms of BaP are known to be the key players involved in the BaP mediated tumor initiation process by leading to the formation of DNA adducts (4).
One of the factors important for the development of cancer is chronic inflammation (5). Pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) are known to contribute not only to tumor promotion and progression, but also to tumor invasiveness, angiogenesis, and metastasis (6). In addition, detoxification enzymes, particularly superoxide dismutases (SOD), are highly expressed in human solid tumors (7).
Heat shock proteins (HSPs) are chaperone proteins that protect living cells against injury-inducing stimuli. Heat shock proteins derived from tumors are capable of eliciting an anticancer immune response. The expression of glucose regulating proteins 78 (GRP78), a major endoplasmic reticulum chaperone with calcium binding and antiapoptotic properties, is increased in cancer cells, and GRP78 has been shown to promote tumor proliferation, survival, and metastasis (8). In addition, Hsp90, a molecular chaperone that is responsible for helping proteins to fold correctly in order to achieve their functional conformation, is highly expressed in cancer cells (9).
In this study, we established a hepatic tumor animal model by injecting rat hepatoma N1-S1 cells into healthy Sprague-Dawley rats. Concomitant injection of BaP induced the proliferation of cancer cells and adipocytes. Histopathological changes in hepatic tumors induced by N1-S1 cells and BaP, separately or in combination with N1-S1 cells were evaluated. Expression of oxidative markers such as HSPs, pro-inflammatory cytokines, and antioxidant enzymes were also examined to evaluate the synergistic effects of BaP on oxidative damage associated with carcinogenesis.
BaP, benzyl alcohol, and all other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Rat hepatoma N1-S1 cells were purchased from American Type Culture Collection (ATCC, Manassas, MA, USA). N1-S1 cells were routinely cultured in MEM + 10% FBS, 4 mM glutamine, 50 µg/mL streptomycin and 100 IU/mL penicillin G. Cells were grown at 37℃ under a 5% CO2 atmosphere. Cells utilized for experiments were obtained under exponential growth conditions.
Male Sprague-Dawley rats (173 ± 5 g) were obtained from Charles River Laboratories, Inc. (Wilmington, MA, USA). Rats were housed under standard laboratory conditions (temperature 24 ± 2℃; humidity 50 ± 10%, 12-hr day/night cycles). Prior to experiments, animals were allowed to acclimatize to the facility for one week, and were provided a standard chow diet and drinking water ad libitum. Animals were six weeks old on the first day of the exposure study.
Twenty rats (six weeks old, five per group) were divided into the following four groups: 1) control group (vehicle, corn oil only), 2) BaP exposure group (200 mg/kg body weight, intraperitoneal injection), 3) N1-S1 group (1 × 107 cells injected directly into the liver) and 4) concomitant BaP and N1-S1 group (N1-S1 intrahepatic injection followed by BaP at 200 mg/kg body weight, intraperitoneal injection). Rats in the non-BaP-treated groups and non-N1-S1-treated groups were injected with either corn oil or culture media, respectively. After four weeks of injections, the rats were sacrificed and blood samples (EDTA-treated whole blood) were collected by cardiac puncture. Whole blood was centrifuged at 1,500 g at 4℃ for 20 min and the resulting plasma was aliquoted and stored at -70℃ for later analysis. This study was conducted from September, 2006 to December, 2008. The procedures for experiments and animal care were approved by the institutional animal care and use committee of Korea University (approval date: Aug. 1, 2008).
Resected right hepatic lobes including tumors were sliced at a thickness of 3 mm, and fixed immediately in a neutral formalin solution, after which paraffin blocks were prepared and cut at a thickness of 4-6 µm. Hematoxylin and eosin staining was performed for histologic evaluation.
Levels of protein oxidation were evaluated by western blot analysis using an anti-dinitrophenylhydrazine antibody. Protein content in the plasma was first determined using the Bradford method, and 10 µg of total protein was then separated electrophoretically on a 10% polyacrylamide gel. Separated proteins were then transferred onto PVDF membranes using an electrophoretic transfer system (Amersham Biosciences, Piscataway, NJ, USA). Non-specific binding sites were blocked for 3 hr in 0.2% casein in PBS. Thereafter, the membrane was incubated for 1 hr with anti-dinitrophenylhydrazine antibody (1:4,000; Invitrogen, Carlsbad, CA, USA) in 0.2% casein. After being washed three times with PBS, the membrane was developed with enhanced chemiluminescence reagents (Amersham Biosciences). Densitometry was performed using a Bio-Rad (Hercules, CA, USA) 700 flatbed scanner with Molecular Analyst software (Bio-Rad). Briefly, the films and membranes were scanned at 600 dots per inch using light transmittance, and pixel volume analysis was performed on the appropriate bands.
Plasma collected by centrifugation of whole blood as described above was mixed with Laemmli sample buffer and boiled in a boiling water bath for 10 min. The resulting samples were then run on 7.5% and 12% sodium dodecyl sulfate-polyacrylamide gels and transferred to Immobilon membranes (Millipore, Billerica, MA, USA). The following antibodies were used for immunodetection: goat anti-mouse Hsp90 (1:1,000; BD Bioscience, San Jose, CA, USA), goat anti-rabbit GRP78 (1:600; Abcam, Cambridge, UK), goat anti-rabbit Hsp27 (1:1,000; Cell Signaling, Danvers, MA, USA), donkey anti-goat SOD-1 (1:1,000; Santa Cruz, Santa Cruz, CA, USA), goat anti-rabbit GST (1:1,000; Santa Cruz), donkey anti-goat TNF-α (1:1,000; Santa Cruz), donkey anti-goat IL-6 (1:1,000; Santa Cruz), goat anti-rabbit IL-1β (1:1,000; Santa Cruz), goat anti-mouse α-tubulin (1:20,000, clone DM1A; Sigma-Aldrich). Goat anti-rabbit, goat anti-mouse, and donkey anti-goat secondary antibodies conjugated to horseradish peroxidase (1:2,500; Santa Cruz) followed by enhanced chemiluminescence reagents (Amersham Biosciences) were used to detect target proteins. Densitometric values were normalized using α-tubulin as an internal control. Scanning of the western blots showed the curve to be linear in the range used for each antibody.
Data in the text and figures is expressed as the mean ± SD. All statistic analyses were conducted using SAS version 9.1. We used the analysis of variance (ANOVA) method with Duncan's and Tukey's test to identify differences between the exposure and control groups. Differences with a P < 0.05 were considered statistically significant.
To develop a hepatic tumor model in rats, rats in experimental group received liver injection of either rat hepatoma N1-S1 cells directly or with BaP (200 mg/kg body weight, IP) and N1-S1 cells. In the control group, rats were intraperitoneally injected with either vehicle or BaP. None of the rats injected with either vehicle or BaP developed hepatic tumors. In contrast, rats injected with N1-S1 cells or both BaP and N1-S1 cells developed hepatic tumors at the injection sites (Fig. 1). Tumor sizes varied from 1.8 mm to 8.54 mm in diameter with an average size of 8.54 ± 4.96 mm for the N1-S1 cell injected group and 7.01 ± 3.14 mm in the BaP and N1-S1 cell injected group, a difference that was not statistically significant. Most of the tumors exhibited a single sharply demarcated margin with multinodular appearance (Fig. 1C), while two tumors from rats injected with N1-S1 cells had small satellite nodules (Fig. 1D). Histological findings for all tumors were similar (Fig. 2). Specifically, tumor cells were composed of large anaplastic cancer cells with variable tumoral necrosis (Fig. 2A, B). Dystrophic calcifications were also frequently observed. Within all tumors, thin or thick fibrous bands were apparent upon examination with higher magnification. Further, the extent of tumoral necrosis was dependent on tumor sizes. Also observed was a small tumor 0.4 cm in diameter with tumoral necrotic areas, associated fibrosis, and reactive inflammation (Fig. 2C). Dystrophic calcifications and reactive foreign body giant cells were also frequently observed (Fig. 2D).
In order to investigate the effects of BaP on the expression of oxidative markers in hepatic tumors induced by rat hepatoma N1-S1 cells, we examined changes in the abundance of HSPs, oxidative stress as indicated by detoxification enzymes and protein oxidation, and expression levels of cytokines. First, plasma samples collected from rats that developed hepatic tumors after injection of N1-S1 cells or concomitant injection of BaP and N1-S1 cells were analyzed for changes in expression of HSPs compared to vehicle-treated or BaP-treated rats by western blot analysis (Fig. 3). Vehicle-treated control rats exhibited relatively low expression levels of Hsp27, however, for BaP-injected rats, levels of Hsp27 in plasma were significantly higher than in the controls. Likewise, rats with cell-induced hepatic tumors showed significantly higher levels of Hsp27 in plasma compared to vehicle-treated controls. In rats with tumors induced by injection of BaP and N1-S1 cells, the levels of Hsp27 in plasma were significantly higher compared to levels in both the vehicle-treated control and BaP-injected rats, as well as compared to those with cell-induced hepatic tumors.
Levels of GRP78 were evaluated by western blot analysis. Levels of GRP78, a member of the Hsp70 family, in BaP-treated rats were higher than those of vehicle-treated control rats, however, this difference was not statistically significant. Conversely, rats that developed N1-S1 cell-induced hepatic tumors exhibited significantly higher levels of GRP78 when compared to vehicle-treated controls. Levels of GRP78 in rats that developed hepatic tumors induced by combined injection of BaP and N1-S1 cells were also higher than in control rats, but were not significantly different from hepatic tumors induced by injection of N1-S1 cells alone (Fig. 3C).
Lastly, protein levels of Hsp90 were also investigated by western blot analysis. All experimental groups showed similar levels of Hsp90 and were not significantly different from each other (Fig. 3D).
The effects of BaP on the antioxidant enzymes SOD-1 and glutathione S-transferase (GST) were evaluated by western blot analysis (Fig. 4A-C). BaP-injected rats showed significantly higher levels of SOD-1 in plasma compared to vehicle-treated controls. On the other hand, levels of SOD-1 in rats with cell-induced hepatic tumors were higher than the vehicle-treated controls, although the difference was not statistically significant. In contrast, rats with hepatic tumors induced by concomitant injection of BaP and N1-S1 cells had significantly higher plasma levels of SOD-1 than vehicle-treated controls, BaP-injected rats, and rats with N1-S1 cell only-induced hepatic tumors.
The levels of GST in the plasma of control rats were significantly lower than in BaP-treated rats, rats with cell-induced hepatic tumors, and rats with hepatic tumors induced by injection of both N1-S1 cells and BaP. However, the levels of GST in rats treated with BaP, rats with cell-induced hepatic tumors, or rats with hepatic tumors induced by injection of both BaP and N1-S1 cells were not significantly different from one another.
Levels of protein oxidation were evaluated by western blot analysis using an anti-dinitrophenylhydrazine antibody (Fig. 4D, E). Vehicle-treated control rats exhibited relatively low levels of protein oxidation, but the injection of BaP only significantly increased the levels of protein oxidation compared to vehicle-treated control rats. Rats with cell-induced hepatic tumors and rats with hepatic tumors induced by injection of both BaP and N1-S1 cells also exhibited a significant increase in protein oxidation compared to vehicle-treated control rats. The levels of protein oxidation in rats treated with BaP, rats with cell-induced hepatic tumors or rats with hepatic tumors induced by injection of both BaP and N1-S1 cells were not significantly different from one another.
The expression levels of cytokines such as TNF-α, IL-1β and IL-6 were evaluated by western blot analysis (Fig. 5). Plasma from vehicle-treated control rats showed relatively low levels of TNF-α. However, rats treated with BaP exhibited significantly increased plasma levels of TNF-α compared to vehicle-treated control rats. Rats with cell-induced hepatic tumors showed a significant increase of TNF-α compared to vehicle-treated control rats, although the difference was not significant when compared with BaP-injected rats. Likewise, rats with hepatic tumors induced by injection of both BaP and N1-S1 cells showed a significant increase of TNF-α in plasma compared to vehicle-treated controls, BaP-injected rats, and rats with cell-induced hepatic tumors.
Treatment with BaP significantly led to a significant increase in the expression levels of IL-6 when compared to control rats. Rats that developed hepatic tumors after injection of N1-S1 cells also showed a significant increase in IL-6 when compared to controls and BaP-treated rats. Conversely, rats with hepatic tumors induced by the injection of both BaP and N1-S1 cells had significantly increased levels of IL-6 when compared to control rats, but not when compared to BaP-treated rats or rats with N1-S1 cell-induced hepatic tumors.
Lastly, the treatment of rats with BaP did not appear to increase the levels of IL-1β in plasma. Specifically, hepatic tumors induced by the injection of cells or by injection of both BaP and N1-S1 cells did not alter the expression of IL-1β compared with controls.
BaP is one of PAHs that humans are primarily exposed to through dietary intake of grilled or smoked meat. BaP is known to cause harmful effects on cell development, growth and survival via an increase of oxidative stress. Therefore, in this study, we did not investigate the carcinogenic effects of BaP, but rather the possible synergistic effects of BaP on the expression of oxidative markers in a tumor model developed by injection of rat hepatoma N1-S1 cells into healthy rats. Histological examination revealed that the incidence of hepatic tumors induced by combined injection of both BaP and N1-S1 cells did not differ significantly when compared with tumors induced by N1-S1 cells alone. However, examination of plasma from these two groups revealed that there were significant increases in the levels of HSPs, antioxidant enzymes, and pro-inflammatory cytokines in rats with hepatic tumors induced by combined injection of both BaP and N1-S1 cells when compared with tumors from rats injected with N1-S1 cells alone.
BaP is one of the most carcinogenic polycyclic aromatic hydrocarbons known. The metabolic activation of BaP by cytochrome P450 isoenzymes produces a variety of mutagenic and/or carcinogenic electrophiles. In particular, BPDE, a metabolite of BaP, covalently binds to DNA, RNA, and proteins. BPDE-DNA adducts have been shown to initiate proto-oncogenic ras (10) and may induce various cancers such as breast cancers (11). BaP-quinones are also one of the BaP-metabolites produced by cytochrome P450 isoenzymes. BaP-quinones are highly active and easily undergo one electron redox cycling resulting in the formation of reactive oxygen species such as the superoxide anion, hydrogen peroxide, and hydroxyl radicals (12), all of which have been associated with cell damage and apoptosis (13). The DNA damage and oxidative stress induced by BaP and observed in this study could therefore have been due to metabolites of BaP such as BPDE or BaP-quinones rather than from BaP itself.
Proteomic analysis of hepatocellular carcinomas (HCCs) collected from respective patients revealed an up-regulation of HSPs such as Hsp27, Hsp70 and GRP78, in HCC. Further, up-regulation of Hsp27 and GRP78 were associated with prognostic values such as α-fetoprotein levels and tumor venous infiltration (14). In addition, Feng et al. (15) reported Hsp27 as a potential biomarker for HCC as evaluated by proteome analysis.
Up-regulation of the HSPs described above has been associated with pro-cancer effects. Hsp27 is a member of the small heat-shock protein family, and its expression is increased in renal cell carcinomas (16), prostate cancer (17) and ovarian cancer (18). Specifically, higher expression of Hsp27 is detected in malignant tumors compared with benign tumors in ovarian cancer, and is associated with advanced cancer stages and reduced survival rates (19). Additionally, increased expression of GRP78 is strongly correlated with the progression of hepatocarcinogenesis (20). GRP78 is a member of the Hsp70 family and can be induced by several stimuli such as glucose starvation, hypoxia and endoplasmic reticulum Ca2+ pool depletion (21). GRP78 has been tentatively implicated in the inhibition of apoptosis and tumor progression in mice (22). In our study, rats that developed hepatic tumors following combined injection of BaP and N1-S1 cells exhibited increased expression of Hsp27 and GRP78 compared to the vehicle-treated controls. In particular, Hsp27 was significantly increased in rats injected with both BaP and N1-S1 cells as compared to rats with tumors induced by N1-S1 cells only. These results suggest that the concomitant injection of BaP had a synergistic effect on the development of hepatic tumors induced by N1-S1 cells.
Previously, it was proposed that persistent oxidative stress accounts for a significant amount of the increased levels of DNA damage observed in cancer tissues. Immunohistochemical studies of SOD activity revealed that hepatocarcinogenesis induced by viral infection is closely related to the depletion of SOD in liver tissues (23). Further, HCC mediated by hepatitis C virus infection is associated with increased lipid peroxidation (24). Short term exposure to BaP has also been shown to increase lipid peroxidation in both serum and liver in an animal model (25). Recently, it has been reported that the malignancy of HCC could be enhanced by increased oxidative stress through the activation of telomerase (26). In our study, the antioxidant enzymes SOD and GST as well as levels of protein oxidations in rats with hepatic tumors induced by combined injection of BaP and N1-S1 cells were significantly higher than the vehicle-treated control rats, suggesting the involvement of oxidative stress in hepatocarcinogenesis induced by the latter treatment type. However, BaP did not synergistically increase oxidative stress in hepatic tumors.
Cancer cells may secrete various cytokines. For example, HepG2 cells express interferon-γ (IFN-γ), TNF-α and IL-4. Likewise, hepatitis-B virus-associated hepatoma cells highly express TGF-β2, IL-8 and monocyte chemoattactant protein-1 (27). Cytokines play a role in carcinogenesis by either controlling proliferation and apoptosis, or by regulating antitumor immune response and neovascular angiogenesis (27). In addition, BaP-exposed cells secrete pro-inflammatory cytokines such as IL-1β and IFN-γ. Increased levels of IL-6 have also been reported in rats exposed to BaP (28). However, conflicting results regarding cytokine TNF-α have been reported. Whereas treatment of primary human macrophages with BaP increases the level of TNF-α significantly (29), TNF-α secretion is unchanged in the murine macrophage cell line RAW 264.7 (30). These contradictory results may be due to species or cell type-specific differences.
In summary, we developed a hepatic tumor animal model by injecting healthy rats with rat hepatoma N1-S1 cells. Injection of N1-S1 cells and concomitant injection of BaP and N1-S1 both resulted in the formation of hepatic tumors at the injection site, and evaluation of the plasma from these two groups revealed significantly increased levels of HSPs such as Hsp27 and GRP78 when compared with controls. Hsp27 in rats with hepatic tumors induced by concomitant injection of BaP and N1-S1 cells was also significantly higher than rats with hepatic tumors induced by the injection of N1-S1 cells alone. Similarly, the levels of antioxidant enzymes such as SOD and GST, as well as cytokines IL-6 and TNF-α were significantly higher in rats with hepatic tumors induced by combined injection of both BaP and N1-S1 cells than in control rats. The expression levels of SOD and TNF-α were also significantly higher in rats with hepatic tumors induced by combined injection of both BaP and N1-S1 cells than in rats with hepatic tumors induced by injection of N1-S1 cells. These results suggest that BaP induces synergistic effects on the expression of several oxidative stress markers, namely, Hsp27, antioxidant enzyme SOD, and cytokine TNF-α in hepatic tumors induced by the injection of N1-S1 cells.
Figures and Tables
Fig. 1
Development of tumors in rats injected with N1-S1 cells. Rats injected with N1-S1 cells were sacrificed one, two, four weeks after injection. (A) No tumors are observed in rats injected with vehicle controls. (B) Small tumors are found one week after injection of N1-S1 cells. (C) Tumors two weeks after injection of N1-S1 cells. A well-defined tumor mass with a multinodular appearance is found. (D) Tumors four weeks after injection of N1-S1 cells. The main tumor exhibits a multinodular appearance with depressed septations. Two satellite lesions are noted. Microscopically, all tumors revealed similar histology.
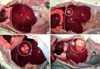
Fig. 2
Histology of tumors induced by concomitant injection of BaP plus N1-S1 cells. Resected livers were fixed in formalin, and sections were stained with hematoxylin and eosin. (A) Most of tumors shows tumoral necrosis (H&E, × 100). (B) Tumors are composed of anaplastic round cells with pushing borders (H&E, × 200), including (C) a small 0.4 cm diameter tumors exhibiting tumoral necrosis associated with extensive fibrosis (H&E, × 100). (D) In necrotic areas, dystrophic calcifications, fibrosis and foreign body type giant cell reaction are observed (H&E, × 200).
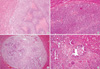
Fig. 3
Expression levels of HSPs in rats with N1-S1 cells or concomitant injection of BaP and N1-S1 cells. Plasma collected from rats with hepatic tumors was evaluated for HSPs and was compared to control-injected and BaP-injected rats. (A) Western blot analysis of HSPs was performed using anti-Hsp27, Hsp90 and GRP78 antibodies. Equal amounts of total protein were loaded into each lane. (B-D) Graphs illustrating the changes in the levels of Hsp27, Hsp90, and GRP78 in the plasma from control-injected rats, BaP-injected rats, and rats with hepatic tumors induced by injection of N1-S1cells or concomitant injection of BaP and N1-S1 cells. Densitometric values are expressed as a ratio to control-injected rats. All data represent the mean ± SD of five different rats. P < 0.05 compared to the control rats, P < 0.05 compared to BaP-injected rats, P < 0.05 compared to rats with N1-S1 cell-induced hepatic tumors, P < 0.05 compared to rats with hepatic tumors induced by concomitant injection of BaP and N1-S1 cells. †, ‡, §, significant difference between control and BaP, N1-S1, and N1-S1 + BaP groups. *, significantly different from control; †, significantly different from BaP; ‡, significantly different from N1-S1; §, significantly different from N1-S1 + BaP.

Fig. 4
Levels of antioxidant enzymes and protein oxidation in rats with hepatic tumors induced by injection of N1-S1 cells or concomitant injection of BaP and N1-S1 cells. Plasma collected from rats with hepatic tumors was evaluated for heat shock proteins and was compared to controls and BaP-injected rats. (A) Western blot analysis of antioxidant enzymes was performed using anti-SOD-1 and GST antibodies. Equal amounts of total protein were loaded into each lane. (B, C) Graphs showing changes in the levels of SOD-1 and GST in plasma from controls, BaP-injected rats, or rats with hepatic tumors induced by injection of N1-S1 cells or concomitant injection of BaP and N1-S1 cells. (D) Western blot analysis of carbonyl content was performed using an anti-dinitrophenylhydrazine antibody. Equal amounts of total protein were loaded into each lane. Arrow: a significant increase in protein oxidation compared to vehicle-treated control rats. (E) Graph showing changes in the levels of protein oxidation in plasma from controls, BaP-injected rats, or rats with hepatic tumors induced by injection of N1-S1 cells or concomitant injection of BaP and N1-S1 cells. Densitometric values are expressed as a ratio to control rats. All data represent the mean ± SD of five different rats. P < 0.05 compared to the control rats, P < 0.05 compared to BaP-injected rats, P < 0.05 compared to rats with N1-S1 cell-induced hepatic tumors, P < 0.05 compared to rats with hepatic tumors induced by concomitant injection of BaP and N1-S1 cells. *, significantly different from control; †, significantly different from BaP; ‡, significantly different from N1-S1; §, significantly different from N1-S1 + BaP.
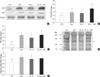
Fig. 5
Expression levels of cytokines in rats with hepatic tumors induced by injection of N1-S1 cells or concomitant injection of BaP and N1-S1 cells. Plasma collected from rats with hepatic tumors was evaluated for cytokines and was compared to controls and BaP-injected rats. (A) Western blot analysis of cytokines was performed using anti-TNF-α, IL-1β and IL-6 antibodies. Equal amounts of total protein were loaded into each lane. (B-D) Graphs showing changes in the levels of TNF-α, IL-1β and IL-6 in plasma from controls, BaP-injected rats or rats with hepatic tumors induced by injection of N1-S1 cells or concomitant injection of BaP and N1-S1 cells. Densitometric values are expressed as a ratio to control rats. All data represent the mean ± SD of five different rats. P < 0.05 compared to the control rats, P < 0.05 compared to BaP-injected rats, P < 0.05 compared to rats with N1-S1 cell-induced hepatic tumors, P < 0.05 compared to rats with hepatic tumors induced by concomitant injection of BaP and N1-S1 cells. *, significantly different from control; †, significantly different from BaP; ‡, significantly different from N1-S1; §, significantly different from N1-S1 + BaP.

AUTHOR SUMMARY
Effects of Benzo(a)pyrene on the Expression of Heat Shock Proteins, Pro-inflammatory Cytokines and Antioxidant Enzymes in Hepatic Tumors Induced by Rat Hepatoma N1-S1 Cells
Zhi Zheng, So-Young Park, Min Lee, Sohee Phark, Nam Hee Won, Hyung-Sik Kang, and Donggeun Sul
Benzo(a)pyrene (BaP) is a polycyclic aromatic hydrocarbon that causes harmful oxidative effects on cells through lipid peroxidation and oxidative DNA damage. In this study, we injected rat hepatoma N1-S1 cells into healthy rats and examined changes in heat shock proteins (HSP), antioxidant enzymes, pro-inflammatory cytokines and oxidative stress levels. Injection of N1-S1 cells or concomitant injection of BaP and N1-S1 cells resulted in the formation of hepatic tumors at the injection site. Evaluation of rat plasma reveals that hepatic tumors induced by BaP and N1-S1 cells expresses higher levels of Hsp27, superoxide dismutase, and tumor necrosis factor-α when compared to those induced by N1-S1 cells only. This study suggest that BaP exerts synergistic effects on the expression of HSP, cytokines and antioxidant enzymes in hepatic tumors induced by rat hepatoma N1-S1 cells.
References
1. The International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans: Polynuclear Aromatic Compounds, Part 1, Chemical, Environmental and Experimental Data. 1998. Lyon: The International Agency for Research on Cancer (IARC).
2. Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol. 2005. 206:73–93.
3. Gelboin HV. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980. 60:1107–1166.
4. Lee BM, Kwack SJ, Kim HS. Age-related changes in oxidative DNA damage and benzo(a)pyrene diolepoxide-I (BPDE-I)-DNA adduct levels in human stomach. J Toxicol Environ Health A. 2005. 68:1599–1610.
5. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002. 420:860–867.
6. Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer. 2006. 42:745–750.
7. Kahlos K, Soini Y, Pääkkö P, Säily M, Linnainmaa K, Kinnula VL. Proliferation, apoptosis, and manganese superoxide dismutase in malignant mesothelioma. Int J Cancer. 2000. 88:37–43.
8. Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007. 67:3496–3499.
9. Drysdale MJ, Brough PA, Massey A, Jensen MR, Schoepfer J. Targeting Hsp90 for the treatment of cancer. Curr Opin Drug Discov Devel. 2006. 9:483–495.
10. Marshall CJ, Vousden KH, Phillips DH. Activation of c-Ha-ras-1 proto-oncogene by in vitro modification with a chemical carcinogen, benzo(a) pyrene diol-epoxide. Nature. 1984. 310:586–589.
11. Rundle A, Tang D, Hibshoosh H, Estabrook A, Schnabel F, Cao W, Grumet S, Perera FP. The relationship between genetic damage from polycyclic aromatic hydrocarbons in breast tissue and breast cancer. Carcinogenesis. 2000. 21:1281–1289.
12. Flowers L, Ohnishi ST, Penning TM. DNA strand scission by polycyclic aromatic hydrocarbon o-quinones: role of reactive oxygen species, Cu(II)/Cu(I) redox cycling, and o-semiquinone anion radicals. Biochemistry. 1997. 36:8640–8648.
13. Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000. 13:135–160.
14. Luk JM, Lam CT, Siu AF, Lam BY, Ng IO, Hu MY, Che CM, Fan ST. Proteomic profiling of hepatocellular carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27, Hsp70, GRP78) up-regulation and their associated prognostic values. Proteomics. 2006. 6:1049–1057.
15. Feng JT, Liu YK, Song HY, Dai Z, Qin LX, Almofti MR, Fang CY, Lu HJ, Yang PY, Tang ZY. Heat-shock protein 27: a potential biomarker for hepatocellular carcinoma identified by serum proteome analysis. Proteomics. 2005. 5:4581–4588.
16. Sarto C, Valsecchi C, Magni F, Tremolada L, Arizzi C, Cordani N, Casellato S, Doro G, Favini P, Perego RA, Raimondo F, Ferrero S, Mocarelli P, Galli-Kienle M. Expression of heat shock protein 27 in human renal cell carcinoma. Proteomics. 2004. 4:2252–2260.
17. Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000. 60:7099–7105.
18. Langdon SP, Rabiasz GJ, Hirst GL, King RJ, Hawkins RA, Smyth JF, Miller WR. Expression of the heat shock protein HSP27 in human ovarian cancer. Clin Cancer Res. 1995. 1:1603–1609.
19. Mehlen P, Mehlen A, Godet J, Arrigo AP. hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem. 1997. 272:31657–31665.
20. Lim SO, Park SG, Yoo JH, Park YM, Kim HJ, Jang KT, Cho JW, Yoo BC, Jung GH, Park CK. Expression of heat shock proteins (HSP27, HSP60, HSP70, HSP90, GRP78, GRP94) in hepatitis B virus-related hepatocellular carcinomas and dysplastic nodules. World J Gastroenterol. 2005. 11:2072–2079.
21. Miyake H, Hara I, Arakawa S, Kamidono S. Stress protein GRP78 prevents apoptosis induced by calcium ionophore, ionomycin, but not by glycosylation inhibitor, tunicamycin, in human prostate cancer cells. J Cell Biochem. 2000. 77:396–408.
22. Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci U S A. 1996. 93:7690–7694.
23. Inagaki T, Katoh K, Takiya S, Ikuta K, Kobayashi S, Suzuki M, Fukuzawa Y, Ayakawa T, Shimizu K, Tagaya T, Katoh K. Relationship between superoxide dismutase (SOD) and viral liver diseases. Gastroenterol Jpn. 1992. 27:382–389.
24. Shimizu I, Inoue H, Yano M, Shinomiya H, Wada S, Tsuji Y, Tsutsui A, Okamura S, Shibata H, Ito S. Estrogen receptor levels and lipid peroxidation in hepatocellular carcinoma with hepatitis C virus infection. Liver. 2001. 21:342–349.
25. Lakshmi B, Ajith TA, Jose N, Janardhanan KK. Antimutagenic activity of methanolic extract of Ganoderma lucidum and its effect on hepatic damage caused by benzo[a]pyrene. J Ethnopharmacol. 2006. 107:297–303.
26. Nishikawa T, Nakajima T, Katagishi T, Okada Y, Jo M, Kagawa K, Okanoue T, Itoh Y, Yoshikawa T. Oxidative stress may enhance the malignant potential of human hepatocellular carcinoma by telomerase activation. Liver Int. 2009. 29:846–856.
27. Shin EC, Choi YH, Kim JS, Kim SJ, Park JH. Expression patterns of cytokines and chemokines genes in human hepatoma cells. Yonsei Med J. 2002. 43:657–664.
28. Garcon G, Campion J, Hannothiaux MH, Boutin AC, Venembre P, Balduyck M, Haguenoer JM, Shirali P. Modification of the proteinase/anti-proteinase balance in the respiratory tract of Sprague-Dawley rats after single intratracheal instillation of benzo[A]pyrene-coated onto Fe(2)O(3) particles. J Appl Toxicol. 2000. 20:265–271.
29. Lecureur V, Ferrec EL, N'Diaye M, Vee ML, Gardyn C, Gilot D, Fardel O. ERK-dependent induction of TNFalpha expression by the environmental contaminant benzo(a)pyrene in primary human macrophages. FEBS Lett. 2005. 579:1904–1910.
30. Chin BY, Choi ME, Burdick MD, Strieter RM, Risby TH, Choi AM. Induction of apoptosis by particulate matter: role of TNF-alpha and MAPK. Am J Physiol. 1998. 275:L942–L949.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download