Abstract
Methylenetetrahydrofolate reductase (MTHFR), a critical enzyme in folate metabolism, plays an important role in DNA methylation. It has been suggested that abnormal DNA methylation contributes to the pathogenesis of schizophrenia and congenital anomalies. The previous findings regarding the genetic relationship between MTHFR and schizophrenia are controversial. This study investigated the association of the two functional polymorphisms of MTHFR, C677T and A1298C, with the risk for schizophrenia. Furthermore, we conducted an updated meta-analysis on the two polymorphisms. In addition, we investigated the relationship between the polymorphisms and minor physical anomaly (MPA), which may represent neurodevelopmental aberrations in 201 schizophrenia patients and 350 normal control subjects. There was no significant association between either of the two polymorphisms and the risk of schizophrenia (chi-square = 0.001, df = 1, P = 0.971 for C677T; chi-square = 1.319, df = 1, P = 0.251 for A1298C). However, in meta-analysis, the C677T polymorphism showed a significant association in the combined and Asian populations (OR = 1.13, P = 0.005; OR = 1.21, P = 0.011, respectively) but not in the Korean and Caucasian populations alone. Neither polymorphism was associated with MPAs measured by the Waldrop scale (chi-square = 2.513, df = 2, P = 0.285). In conclusion, the present findings suggest that in the Korean population, the MTHFR polymorphisms are unlikely to be associated with the risk for schizophrenia and neurodevelopmental abnormalities related to schizophrenia.
Methylenetetrahydrofolate reductase (MTHFR) is an enzyme that reduces 10-methylenetetrahydrofolate (10-MTHF) to 5-methylenetetrahydrofolate (5-MTHF). 5-MTHF is a cofactor for the remethylation of homocysteine to convert it to S-adenosyl methionine, which is methyl group donor for DNA methyltransferases (1). It has long been suggested that abnormal methylation contributes to the pathogenesis of schizophrenia through downstream methylation-dependent processes (2) and through the alteration of neurotransmitters, including dopamine, serotonin, GABA, etc. (3).
In addition, it has been suggested that MTHFR is related to the pathophysiology of abnormal congenital development. Folic acid supplementation during early pregnancy reduces some congenital anomalies, such as neural tube defects, oro-facial clefts and heart defects (4). Although the exact mechanism of how folic acid metabolism affects fetal development is not known, several genetic studies on the association between folate metabolism and congenial anomalies have been carried out (5, 6). As an influential enzyme in folate metabolism, the MTHFR gene is one of the candidates that have been studied. Several congenital central nervous system anomalies, such as neural tube defects, have been reported to be related to MTHFR gene polymorphisms (5, 6).
Patients with schizophrenia have been reported to have higher incidence of minor physical anomalies (MPAs) than control groups (7). MPAs are minor abnormalities of morphogenesis that lead to subtle alterations in the development of various bodily structures in the area of the face, eyes, ears, mouth, and feet. The presence of MPAs in patients with schizophrenia has been reported as a stable manifestation (7, 8) and it has been suggested that they reflect the genetic vulnerability for schizophrenia (8, 9). MPAs also meet the criteria of endophenotype in schizophrenia (8). Although their direct relationship with psychiatric symptoms has not been established, from a developmental perspective both the external tissues and the central nervous system share a common ectoderm origin. Therefore, MPAs seen in schizophrenic patients may have a common genetic background with neural tube defects, which are related to MTHFR polymorphisms. Accordingly, MTHFR is a good candidate marker to explain the relationship between schizophrenia and MPAs.
Two common single nucleotide polymorphisms in MTHFR gene have been reported. One is a C/T transition at nucleotide 677 in exon 4, and the other is an A/C transversion in exon 7 at position 1298. Both mutations are functional and result in a reduction in the enzyme's activity (10). The association of MTHFR gene polymorphisms at C677T and A1298C with schizophrenia has been studied in various populations. Some studies have reported an association of the single nucleotide polymorphisms (SNPs) with schizophrenia (11-18), but others have reported no association (19-27). Several meta-analyses continue to indicate the positive association of the C677T polymorphism with schizophrenia (1, 19, 28-30). The positive association between the C677T polymorphism and schizophrenia was suggested to occur mainly in East Asians (1, 16), which suggests a higher risk for schizophrenia from the MTHFR gene in Asian populations. In the Korean population, Lee et al. (16) reported a positive association of the C677T polymorphism and schizophrenia. However, Kang et al. (20) recently reported no association with the C677T polymorphism. Therefore, further investigation is needed into the relationship between the MTHFR polymorphism and schizophrenia in the Korean population.
In this study, we first examined the association of the genotype and the allelic frequencies of the MTHFR 677C > T and 1298 A > C polymorphisms with the risk for schizophrenia in the Korean population. Then we performed an updated meta-analysis on the two polymorphisms in Korean, Asian, and Caucasian populations as well as the three populations combined. Finally, we examined whether the genotypic frequencies of the MTHFR 677C > T and 1298 A > C polymorphisms were associated with the scores from the Waldrop scale, which was developed to measure MPAs (9, 31).
All patients with schizophrenia were recruited from the Seoul National University Hospital in Korea between March 2005 and September 2010. All patients fulfilled the diagnostic criteria from the DSM-IV for schizophrenia and were interviewed individually by trained nurses using a Korean-translated version of the Diagnostic Interview for Genetic Studies (DIGS) (32). Consensus diagnostic meetings were held regularly to evaluate the participants' final diagnosis. The interview material from the DIGS and the hospital records were the major sources used to re-evaluate the diagnoses. The subjects who had a history of having any kind of organic abnormality of the brain, alcohol-related mental problem, drug abuse, or other physical illnesses that potentially manifested as psychiatric symptoms were not included in this study. The subjects included in the final analysis included 201 patients (average age = 32.89 ± 7.76 yr), 133 males (average age = 31.75 ± 6.91 yr) and 68 females (average age = 35.10 ± 8.85 yr).
Normal controls without psychiatric disorders were randomly recruited from hospital staff members and college students who volunteered for participation. After a brief interview by a psychiatrist, the subjects with a past history or current evidence of psychiatric illness, organic mental disorders, the abuse of illegal substances and any medical conditions that might give rise to mental symptoms were excluded. In addition, control subjects who had any 1st degree relatives with suspected psychiatric illness were also excluded. The total number of control subjects was 350 (average age = 25.9 ± 6.56 yr), 174 males (average age = 23.38 ± 5.51 yr) and 176 females (average age = 24.58 ± 8.27 yr). The mean (S.D.) age of the patients with schizophrenia was older than that of the controls. All of the subjects with schizophrenia and the control subjects were ethnically Korean.
As described in Joo et al. (9), MPAs were measured with a modified 15-item Waldrop scale for both the patients with schizophrenia and the normal controls. While the original 18-item Waldrop scale was developed to measure MPAs around the head, eyes, ears, mouth, hands, and feet (31), we used a modified 15-item Waldrop scale that excluded three items (two or more hair whorls, soft and pliable ears, and a tongue with smooth-rough spots) that do not contribute to the total score (33). Our previous study showed good inter-rater reliability for the total Waldrop score (intraclass correlation coefficient = 0.59) (33). The MPAs were measured by trained researchers who could not be kept blind with regard to the diagnostic group membership. Patients with total Waldrop scores above 5 were defined as the high MPA subgroup (9). The data for the Waldrop total was present in 217 of 350 control group members and 122 of 201 patients.
DNA was extracted from blood samples using a DNA isolation kit (Roche, Basel, Switzerland). Genotyping for the MTHFR C677T and A1298C polymorphisms was performed using the TaqMan™ method (Applied Biosystems). The primer and probe sets were provided by Applied Biosystems (Foster City, CA, USA). The Taq-Man probes contain a reporter dye at the 5' end and a quencher dye at the 3' end. The quencher dye suppresses the fluorescent signal of the reporter dye. When the probe is hybridized to the target sequence, Taq polymerase, which has a 5'→3' exonuclease activity, cleaves the reporter dye-labeled probes. The fluorescence intensity increases as the amount of freed reporter dye increases. The intensity of the fluorescence was measured using an Applied Biosystems PRISM 7900HT Sequence Detector System. The genotypes were analyzed according to the fluorescence characteristics. The PCR reactions were performed in a 5-µL total volume, which included 1 µL (50 ng) of genomic DNA, 0.1 µL (5 pM/µL) of each probe, 0.15 µL (20 pM/µL) of each primer, 2.5 µL of 2 × Taqman PCR Master Mix, and 1 µL of DW. The thermal conditions were 15 min at 95℃, 40 cycles of 15 sec at 95℃ and 60 sec at 60℃, followed by cooling to 4℃. The data analysis was done using SDS 2.1 software (Applied Biosystems), and these genotyping procedures were done by Seoul Clinical Genomics, Inc.
Hardy-Weinberg equilibrium was tested by a goodness of fit chi-squared test. Contingency chi-squared tests were performed to compare the allele and genotype frequencies between the patients and the normal controls. Logistic regression analysis was performed to estimate the relative risk of the presence of high MPA in relation to SNP genotype using diagnosis and sex as covariates. Haplotype analysis for the phenotype of the affection status and the Waldrop total score and the linkage disequilibrium (LD) calculation between the C677T and A1298C SNPs were done using the software, UNPHASED 3.0.13 (http://www.mrcbsu.cam.ac.uk/personal/frank/software/unphased/).
Before beginning the study, the protocol was reviewed and approved by the institutional review board (IRB) of the Seoul National University Hospital (IRB No. H-0106-080-002). Informed consent was confirmed by the IRB. All participants who signed written informed consent forms were provided with detailed information about the genetic study.
We performed an updated meta-analysis for the C677T and A1298C MTHFR SNPs. For C677T, we included 3 Korean (including this study), 5 Asian (non-Korean), and 14 Caucasian data sets from currently available published data to explore the difference among the ethnic groups (11-13, 15-27, 34-36). For A1298C, 3 Korean (including this study), 2 Asian (non-Korean), and 9 Caucasian studies were included (14, 16-20, 25, 27, 35, 36).
The homogeneity of the included studies was assessed by Cochrane's Q test and the I2 index. The Q statistics suggested that there was significant heterogeneity for C677T (Q = 46.85, P = 0.001) but not for A1298C (Q = 20.57, P = 0.082). The I2 index also suggested moderate heterogeneity for C677T (I2 = 0.552) and weak heterogeneity for A1298C (I2 = 0.368). However, we decided to apply a random-effects model to both C677T and A1298C because the random-effects model is more widely used in genetic association studies and is considered more reasonable than a fixed-effects approach (37). The pooled estimates of the Odds Ratio (OR) were calculated using the DerSimonian-Laird method (38).
The pooled OR was obtained for all of the included studies, and the subgroup ORs were obtained for each of the different ethnic groups (Caucasian, Asian and Korean). The significance of the overall Odds Ratio (OR) was determined using the Z-test. The threshold for statistical significance was set at P < 0.05. The whole analysis was done using MetaAnalyst, a freely available software package that is developed and maintained by Tufts Medical Center (39).
Neither the patients with schizophrenia nor the normal controls were found to deviate from Hardy-Weinberg equilibrium with regard to C677T (P = 0.962 for schizophrenics; P = 0.832 for controls) and A1298C (P = 0.557 for schizophrenics; P = 0.224 for controls).
No significant association was found in the comparison of the patients with schizophrenia and the controls with regard to the allele frequency for both the C677T and the A1298C polymorphisms (chi-square = 0.001, df = 1, P = 0.971; chi-square = 1.319, df = 1, P = 0.251, respectively; Table 1). The genotype frequencies for the patients with schizophrenia were similar to those of the normal controls, resulting in no significant association (chi-square = 0.345, df = 2, P = 0.841; chi-square = 1.611, df = 2, P = 0.447, respectively; Table 1). No significant association for the allele and the genotype frequency was found in the comparisons between males and females (Table 1).
According to the reference sequence information on SNPs from the National Center for Biotechnology Information (NCBI), the two SNPs are about 1900 bps apart (C677T: rs1801133, 11778965 and A1298C: rs1801131, 11777063). The linkage disequilibrium was calculated as r2 = 0.16 and D' = 1 for the combined subjects. The haplotype of the C677T and A1298C polymorphism was not found to have a significant difference for the affection status between the schizophrenia and control subjects (chi-square = 1.531, df = 2, P = 0.465). There was no haplotypic association for the Waldrop total score (chi-square = 0.758, df = 2, P = 0.685), either.
Meta-analysis using the combined data from the Korean, Asian, and Caucasian populations showed a significant association between the C677T SNP and the risk for schizophrenia (OR = 1.13, P = 0.005; Table 2). However, when we meta-analyzed the three populations separately, only the Asian population showed a significant association (OR = 1.21, P = 0.011 for Asians, including Korean; OR = 1.29, P = 0.023 for Asians, excluding Korean). No significant association was obtained for the Korean and Caucasian populations, (OR = 1.12, P = 0.212 for Koreans; OR = 1.09, P = 0.106 for Caucasians). For the A1298C polymorphism, meta-analysis using the combined populations showed no association between the polymorphism and schizophrenia (OR = 1.09, P = 0.071; Table 3). When we meta-analyzed the three populations separately, none of the populations showed a significant association (OR = 0.97, P = 0.384 for Koreans; OR = 1.10, P = 0.258 for Asians, including Korean; OR = 1.08, P = 0.124 for Caucasians) except for the Asian, excluding Koreans, population (OR = 1.32, P = 0.007).
The total Waldrop score was significantly higher in the patients with schizophrenia than in the normal controls (4.70 ± 1.85 for schizophrenia, 4.12 ± 1.57 for control, P = 0.009). The difference was significant only in females (4.78 ± 2.04 for schizophrenia, 3.75 ± 1.63 for control, P = 0.007) when we analyzed data separately for males and females. 142 out of 339 subjects was classified as a high MPA subgroup (the Waldrop total score ≥ 5). The calculated odds ratios (ORs) for the presence of high MPAs associated with the genotypes of C677T and A1298C polymorphisms are presented in Table 4. There was no statistically significant association between the Waldrop score and C677T and A1298C polymorphisms in relation to schizophrenia risk and sex.
In this study, we tested the hypothesis that the MTHFR C677T and A1298C polymorphisms are associated with the risk of schizophrenia and MPA manifestations in schizophrenia. Our findings suggest that there is no association of the two functional polymorphisms with schizophrenia development. The MPAs also showed no association with both polymorphisms.
The previous findings on the genetic relationship between MTHFR and schizophrenia have been controversial. Although our study showed no association between the C677T and A1298C SNPs and the development of schizophrenia, our meta-analysis demonstrated support for the association of C677T with schizophrenia. Previous studies suggested that demographic differences, such as ethnicity, may influence the association of the MTHFR polymorphisms with psychiatric disorders (1, 35). We meta-analyzed the previous case-control studies for C677T by ethnic groups. Although we obtained no association between the C677T polymorphism and schizophrenia in either of the Korean or Caucasian populations, the Asian population showed a significant association. Taken together, ethnicity may influence the effect of MTHFR genetic variations on the risk of developing schizophrenia, although Peerbooms et al. (40) reported no significant moderating effect of ethnicity on major psychiatric disorders, including schizophrenia, bipolar disorder, and major depressive disorder, in their meta-analysis. For A1298C, although some studies reported a positive association with schizophrenia that was of borderline significance (1, 19, 28), more recent meta-analyses have indicated no association of A1298C with schizophrenia (29, 30). Our meta-analysis confirmed that there is no association between the A1298C polymorphism and schizophrenia, and the results from the meta-analyses by ethnic groups were also consistent with the main findings.
The polymorphism, C677T, substitutes an alanine for a valine, and each copy of the variant reduces the folate-related metabolic activity by approximately 35% (41). The serum homocysteine level is also increased by up to 25% with the TT genotype (42). The A1298C polymorphism causes a glutamine to be substituted for an alanine and decreases the enzyme activity to a lesser extent than the C677T allele (10). Therefore, MTHFR polymorphisms can increase serum homocysteine level and as a consequence result in an impairment of DNA methylation. Both of these are known to be risk factors for neuropsychiatric disorder including schizophrenia (43). In a previous study, 45% of schizophrenic patients with hyperhomocysteinemia were reported to have inherent methylation deficiencies (44). Therefore, the MTHFR gene polymorphisms may be related to developmental abnormalities in schizophrenia through mechanisms related to hyperhomocysteinemia and DNA methylation impairment (43). In an animal model, both heterozygous and homozygous MTHFR knockout mice showed decreased DNA methylation in the brain (45). Furthermore, the presence of the MTHFR T allele in the fetus could affect the levels of methylenetetrahydrofolate in circulation, which is involved in the DNA repair capacity and protecting against congenital anomalies, i.e., against clubfoot (46). Overall, these findings suggest that neurodevelopmental abnormalities in schizophrenia could be related to aberrant DNA methylation due to a polymorphism at the MTHFR gene. Developmental defects manifested as MPAs in schizophrenic patients may have a common genetic cause with the neurodevelopmental etiology of schizophrenia, and MTHFR gene polymorphisms could be a crucial candidate for both of them. Nevertheless, our findings do not support a contribution of the MTHFR gene to the manifestation of MPAs and the development of schizophrenia. As far as we know, our study is the only one that has reported the genetic relationship between MTHFR gene variants and MPAs. Because the sample size of the present study is small and there may be other MTHFR polymorphisms that are associated with schizophrenia and developmental anomalies, further studies are still called for with this gene.
The limitation of the present study is that the sample size involved is relatively small. In particular, the number of CC carriers of the A1298C variant was too small. Studies with a larger sample size are warranted to confirm that the A1298C polymorphism is a risk factor for schizophrenia. In addition, although we tried to match the age and sex between the schizophrenia and control groups to avoid population stratification, the mean age of the control group was younger than that of the schizophrenic group. The mean age of the control group is under the age of risk for the development of schizophrenia. This means that the controls still have a chance of developing schizophrenia in the future. However, the lifetime prevalence of schizophrenia is only 1% in the general population. Therefore, this should not significantly influence our results.
In conclusion, the present study provides evidence that the MTHFR C677T and A1298C variants may not influence the risk of schizophrenia development in the Korean population. However, meta-analyses support an association between the C677T variant and schizophrenia, especially in Asian populations. Furthermore, the two polymorphisms may not be associated with the minor physical anomalies in schizophrenia, which have been suggested to be related to the neurodevelopmental etiology of schizophrenia. Additional genetic investigation is needed to further analyze this connection.
Figures and Tables
Table 1
The MTHFR C677T and A1298C allele frequencies in patients with schizophrenia and control subjects

Table 2
Case-control association analysis of the C677T MTHFR polymorphism in each of the populations and the meta-analysis
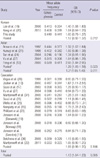
Table 3
Case-control association analysis of the A1298C MTHFR polymorphism in each of the populations and the meta-analysis
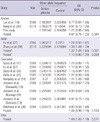
Table 4
Association between the Waldrop score and the genotypes of C677T and A1298C polymorphism at the MTHFR gene

Logistic regression analysis was applied to test for an association between the Waldrop score and the C677T and A1298C polymorphism. Waldrop total scores were measured by the Waldrop scale that was developed to measure minor physical anomalies (9). Patients with Waldrop total score < 5 were defined as the low MPA subgroup, and those ≥ 5 were defined as the high MPA subgroup. OR, odds ratio; CI, confidence interval; MPA, minor physical anomaly.
AUTHOR SUMMARY
No Association of Functional Polymorphisms in Methlylenetetrahydrofolate Reductase and the Risk and Minor Physical Anomalies of Schizophrenia in Korean Population
Su-Gyeong Kim, Joo Yun Song, Eun-Jeong Joo, Seong Hoon Jeong, Se Hyun Kim, Kyu Young Lee, Nam Young Lee, Yong Min Ahn, Yong Sik Kim and Myoung-Sun Roh
We investigated genetic relationship between methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms and the risk and minor physical anomalies in schizophrenia in the Korean population. We found no association between the C677T and A1298C polymorphisms and schizophrenia. However, an updated meta-analysis of the previous reports showed association between the C677T polymorphism and schizophrenia, especially in Asian population. Neither polymorphism was associated with the presence of minor physical anomalies that may represent neurodevelopmental aberration in schizophrenia. Taken together, our findings suggest that the MTHFR polymorphisms are unlikely to contribute to the risk for schizophrenia in the Korean population.
References
1. Zintzaras E. C677T and A1298C methylenetetrahydrofolate reductase gene polymorphisms in schizophrenia, bipolar disorder and depression: a meta-analysis of genetic association studies. Psychiatr Genet. 2006. 16:105–115.
2. Abdolmaleky HM, Smith CL, Faraone SV, Shafa R, Stone W, Glatt SJ, Tsuang MT. Methylomics in psychiatry: Modulation of gene-environment interactions may be through DNA methylation. Am J Med Genet B Neuropsychiatr Genet. 2004. 127B:51–59.
3. Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000. 69:228–232.
4. Shaw GM, Lammer EJ, Wasserman CR, O'Malley CD, Tolarova MM. Risks of orofacial clefts in children born to women using multivitamins containing folic acid periconceptionally. Lancet. 1995. 346:393–396.
5. Shaw GM, Lu W, Zhu H, Yang W, Briggs FB, Carmichael SL, Barcellos LF, Lammer EJ, Finnell RH. 118 SNPs of folate-related genes and risks of spina bifida and conotruncal heart defects. BMC Med Genet. 2009. 10:49.
6. Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol. 2000. 151:862–877.
7. Lawrie SM, Byrne M, Miller P, Hodges A, Clafferty RA, Cunningham Owens DG, Johnstone EC. Neurodevelopmental indices and the development of psychotic symptoms in subjects at high risk of schizophrenia. Br J Psychiatry. 2001. 178:524–530.
8. Compton MT, Walker EF. Physical manifestations of neurodevelopmental disruption: are minor physical anomalies part of the syndrome of schizophrenia? Schizophr Bull. 2009. 35:425–436.
9. Joo EJ, Jeong SH, Ahn YM, Lee KY, Chang Yoon S, Kim EJ, Kim SU, Cho SC, Sik Kim Y. No association found between 158 Val/Met polymorphism of the COMT gene and schizophrenia with minor physical anomalies. Psychiatry Res. 2005. 136:83–91.
10. van der Put NM, Gabreëls F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, van den Heuvel LP, Blom HJ. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet. 1998. 62:1044–1051.
11. Arinami T, Yamada N, Yamakawa-Kobayashi K, Hamaguchi H, Toru M. Methylenetetrahydrofolate reductase variant and schizophrenia/depression. Am J Med Genet. 1997. 74:526–528.
12. Feng LG, Song ZW, Xin F, Hu J. Association of plasma homocysteine and methylenetetrahydrofolate reductase C677T gene variant with schizophrenia: a Chinese Han population-based case-control study. Psychiatry Res. 2009. 168:205–208.
13. Joober R, Benkelfat C, Lal S, Bloom D, Labelle A, Lalonde P, Turecki G, Rozen R, Rouleau GA. Association between the methylenetetrahydrofolate reductase 677C-->T missense mutation and schizophrenia. Mol Psychiatry. 2000. 5:323–326.
14. Kempisty B, Bober A, Luczak M, Czerski P, Szczepankiewicz A, Hauser J, Jagodziński PP. Distribution of 1298A>C polymorphism of methylenetetrahydrofolate reductase gene in patients with bipolar disorder and schizophrenia. Eur Psychiatry. 2007. 22:39–43.
15. Kempisty B, Mostowska A, Górska I, Łuczak M, Czerski P, Szczepankiewicz A, Hauser J, Jagodziński PP. Association of 677C>T polymorphism of methylenetetrahydrofolate reductase (MTHFR) gene with bipolar disorder and schizophrenia. Neurosci Lett. 2006. 400:267–271.
16. Lee YS, Han DH, Jeon CM, Lyoo IK, Na C, Chae SL, Cho SC. Serum homocysteine, folate level and methylenetetrahydrofolate reductase 677, 1298 gene polymorphism in Korean schizophrenic patients. Neuroreport. 2006. 17:743–746.
17. Sazci A, Ergül E, Guzelhan Y, Kaya G, Kara I. Methylenetetrahydrofolate reductase gene polymorphisms in patients with schizophrenia. Brain Res Mol Brain Res. 2003. 117:104–107.
18. Zhang C, Xie B, Du Y, Cheng W, Fang Y, Yu S. Further evidence that methylenetetrahydrofolate reductase A1298C polymorphism is a risk factor for schizophrenia. J Neural Transm. 2010. 117:1115–1117.
19. Jonsson EG, Larsson K, Vares M, Hansen T, Wang AG, Djurovic S, Rønningen KS, Andreassen OA, Agartz I, Werge T, Terenius L, Hall H. Two methylenetetrahydrofolate reductase gene (MTHFR) polymorphisms, schizophrenia and bipolar disorder: an association study. Am J Med Genet B Neuropsychiatr Genet. 2008. 147B:976–982.
20. Kang HJ, Choe BM, Kim SH, Son SR, Lee KM, Kim BG, Hong YS. No Association Between Functional Polymorphisms in COMT and MTHFR and Schizophrenia Risk in Korean Population. Epidemiol Health. 2010. 32:e2010011.
21. Kunugi H, Fukuda R, Hattori M, Kato T, Tatsumi M, Sakai T, Hirose T, Nanko S. C677T polymorphism in methylenetetrahydrofolate reductase gene and psychoses. Mol Psychiatry. 1998. 3:435–437.
22. Muntjewerff JW, Ophoff RA, Buizer-Voskamp JE, Strengman E, den Heijer M. GROUP Consortium. Effects of season of birth and a common MTHFR gene variant on the risk of schizophrenia. Eur Neuropsychopharmacol. 2011. 21:300–305.
23. Philibert R, Gunter T, Hollenbeck N, Adams WJ, Bohle P, Packer H, Sandhu H. No association of the C677T methylenetetrahydrofolate reductase polymorphism with schizophrenia. Psychiatr Genet. 2006. 16:221–223.
24. Tan EC, Chong SA, Lim LC, Chan AO, Teo YY, Tan CH, Mahendran R. Genetic analysis of the thermolabile methylenetetrahydrofolate reductase variant in schizophrenia and mood disorders. Psychiatr Genet. 2004. 14:227–231.
25. Vilella E, Virgos C, Murphy M, Martorell L, Valero J, Simø JM, Joven J, Fernández-Ballart J, Labad A. Further evidence that hyperhomocysteinemia and methylenetetrahydrofolate reductase C677T and A1289C polymorphisms are not risk factors for schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005. 29:1169–1174.
26. Virgos C, Martorell L, Simø JM, Valero J, Figuera L, Joven J, Labad A, Vilella E. Plasma homocysteine and the methylenetetrahydrofolate reductase C677T gene variant: lack of association with schizophrenia. Neuroreport. 1999. 10:2035–2038.
27. Yu L, Li T, Robertson Z, Dean J, Gu NF, Feng GY, Yates P, Sinclair M, Crombie C, Collier DA, Walker N, He L, St Clair D. No association between polymorphisms of methylenetetrahydrofolate reductase gene and schizophrenia in both Chinese and Scottish populations. Mol Psychiatry. 2004. 9:1063–1065.
28. Shi J, Gershon ES, Liu C. Genetic associations with schizophrenia: metaanalyses of 12 candidate genes. Schizophr Res. 2008. 104:96–107.
29. O'Connor MN, Salles II, Cvejic A, Watkins NA, Walker A, Garner SF, Jones CI, Macaulay IC, Steward M, Zwaginga JJ, Bray SL, Dudbridge F, de Bono B, Goodall AH, Deckmyn H, Stemple DL, Ouwehand WH. Bloodomics Consortium. Functional genomics in zebrafish permits rapid characterization of novel platelet membrane proteins. Blood. 2009. 113:4754–4762.
30. Burns P, Gusnanto A, Macaulay IC, Rankin A, Tom B, Langford CF, Dudbridge F, Ouwehand WH, Watkins NA. Bloodomics Consortium. Identification of variation in the platelet transcriptome associated with glycoprotein 6 haplotype. Platelets. 2008. 19:258–267.
31. Guy JD, Majorski LV, Wallace CJ, Guy MP. The incidence of minor physical anomalies in adult male schizophrenics. Schizophr Bull. 1983. 9:571–582.
32. Joo EJ. Development and application of DIGS-K (Diagnostic Interview for Genetic Studies-Korean). Schizophr Clin. 2003. 6:19–23.
33. Joo EJ, Jeong SH, Maeng SJ, Yoon SC, Kim JH, Kim CE, Shin Y, Kim YS. Minor physical anomalies in patients with schizophrenia. J Korean Soc Biol Psychiatry. 2002. 9:140–151.
34. Muntjewerff JW, Hoogendoorn ML, Kahn RS, Sinke RJ, Den Heijer M, Kluijtmans LA, Blom HJ. Hyperhomocysteinemia, methylenetetrahydrofolate reductase 677TT genotype, and the risk for schizophrenia: a Dutch population based case-control study. Am J Med Genet B Neuropsychiatr Genet. 2005. 135B:69–72.
35. Sazci A, Ergul E, Kucukali I, Kara I, Kaya G. Association of the C677T and A1298C polymorphisms of methylenetetrahydrofolate reductase gene with schizophrenia: association is significant in men but not in women. Prog Neuropsychopharmacol Biol Psychiatry. 2005. 29:1113–1123.
36. Betcheva ET, Mushiroda T, Takahashi A, Kubo M, Karachanak SK, Zaharieva IT, Vazharova RV, Dimova II, Milanova VK, Tolev T, Kirov G, Owen MJ, O'Donovan MC, Kamatani N, Nakamura Y, Toncheva DI. Case-control association study of 59 candidate genes reveals the DRD2 SNP rs6277 (C957T) as the only susceptibility factor for schizophrenia in the Bulgarian population. J Hum Genet. 2009. 54:98–107.
37. Kavvoura FK, Ioannidis JP. Methods for meta-analysis in genetic association studies: a review of their potential and pitfalls. Hum Genet. 2008. 123:1–14.
38. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986. 7:177–188.
39. Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009. 9:80.
40. Peerbooms O, Rutten BP, Decoster J, van Os J, Kenis G, De Hert M, van Winkel R. No association between MTHFR C677T or A1298C and age at onset of schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2010. 153B:1362–1363.
41. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, Rozen R. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995. 10:111–113.
42. Jongbloet PH, Verbeek AL, den Heijer M, Roeleveld N. Methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms resulting in suboptimal oocyte maturation: a discussion of folate status, neural tube defects, schizophrenia, and vasculopathy. J Exp Clin Assist Reprod. 2008. 5:5.
43. Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, Jia P, Assadzadeh A, Flanagan J, Schumacher A, Wang SC, Petronis A. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008. 82:696–711.
44. Dudbridge F. Likelihood-based association analysis for nuclear families and unrelated subjects with missing genotype data. Hum Hered. 2008. 66:87–98.
45. Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, Chen MF, Pai A, John SW, Smith RS, Bottiglieri T, Bagley P, Selhub J, Rudnicki MA, James SJ, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001. 10:433–443.
46. Sharp L, Miedzybrodzka Z, Cardy AH, Inglis J, Madrigal L, Barker S, Chesney D, Clark C, Maffulli N. The C677T polymorphism in the methylenetetrahydrofolate reductase gene (MTHFR), maternal use of folic acid supplements, and risk of isolated clubfoot: a case-parent-triad analysis. Am J Epidemiol. 2006. 164:852–861.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download