Abstract
Little is known about hair mineral status in fibromyalgia patients. This study evaluated the characteristics of hair minerals in female patients with fibromyalgia compared with a healthy reference group. Forty-four female patients diagnosed with fibromyalgia according to the American College of Rheumatology criteria were enrolled as the case group. Ageand body mass index-matched data were obtained from 122 control subjects enrolled during visit for a regular health check-up. Hair minerals were analyzed and compared between the two groups. The mean age was 43.7 yr. General characteristics were not different between the two groups. Fibromyalgia patients showed a significantly lower level of calcium (775 µg/g vs 1,093 µg/g), magnesium (52 µg/g vs 72 µg/g), iron (5.9 µg/g vs 7.1 µg/g), copper (28.3 µg/g vs 40.2 µg/g) and manganese (140 ng/g vs 190 ng/g). Calcium, magnesium, iron, and manganese were loaded in the same factor using factor analysis; the mean of this factor was significantly lower in fibromyalgia group in multivariate analysis with adjustment for potential confounders. In conclusion, the concentrations of calcium, magnesium, iron, and manganese in the hair of female patients with fibromyalgia are lower than of controls, even after adjustment of potential confounders.
Fibromyalgia is characterized by chronic generalized pain, muscle tenderness, and fatigue. The etiology and mechanisms of fibromyalgia are not well understood, as is the pattern of high prevalence of fibromyalgia in middle age and subsequent decrease (1). Fibromyalgia is much more prevalent in women than in men (2).
Some hypotheses have been introduced to explain the pathophysiology of fibromyalgia. Explanations for the pain include peripheral muscle involvement (3), central pain mechanism (4), multiple neurotransmitter hypothesis (5, 6), and psychiatric aspects (7, 8). Various trials have been conducted to evaluate these suggested sources, but success has been limited; a longitudinal study reported that about half of fibromyalgia patients were not satisfied their health-related quality of life in spite of various treatments (9). Therefore, a new approach to understand this disease is required.
Several studies have explored the relationship between fibromyalgia and oxidative stress (10, 11). Still other studies investigated elemental composition of patients with fibromyalgia, but they surveyed only in blood and urine samples (12-14). The latter studies, while potentially useful, overlooked the mineral content of hair. Hair mineral assay can be an effective method to deduce intracellular status. Some studies tried to find the characteristics of the elements in some diseases, including fibromyalgia (15-17). The present study investigated the elemental characteristics in the hair of women with fibromyalgia, and compared the data with those from healthy age- and body mass-matched women.
This cross-sectional study was conducted in case-control design. Forty four female patients with fibromyalgia aged 29-57 yr were recruited at the Department of Rheumatology and Department of Family Practice and Community Health, Ajou University Hospital, from March-August, 2010. The diagnosis of fibromyalgia was based on the American College of Rheumatology (ACR) criteria (18). Age- and body mass index (BMI)-matched healthy control female patients without fibromyalgia, who were recruited and provided informed consent during their visit to the health promotion center, Ajou University Hospital, were the reference group. Women who had diabetes, thyroid diseases, rheumatologic disorders, cancers, and psychological problems were excluded from the reference group.
For analysis of hair minerals, all participants were asked not to chemically process their hair (i.e., no dyeing, perms, or frosting) for at least 2 weeks prior to hair sample acquisition. The hair had also to be free of all gels, oils, and hair creams before sample collection. Approximately 300 mg of hair were obtained from four to five different locations of the posterior vertex region of the scalp using stainless steel scissors. The only proximal portion (within 3.8 cm of the root) was used as the sample. The cut hair was placed directly into a clean hair specimen envelope normally provided by the laboratory and then sealed with the envelope's glue flap. The hair samples were not washed for the assays. ICP-Mass Spectrometry (Perkin-Elmer, Waltham, MA, USA) was applied to routine elemental analysis. A licensed and certified clinical laboratory performed all testing in a trace element laboratory clean room environment, utilized the latest microwave temperature-controlled digestion technique.
Height (cm) and weight (0.1 kg units) measurements were taken while each participant was lightly clothed. BMI was calculated with measured height and weight (kg/m2). Waist circumference was measured at the central part between the twelfth rib and iliac crest by a trained nurse in the outpatient clinic of the Department of Family Practice and Community Health.
The amount of each element was recorded per 1 g hair. All data below detection limit were replaced by the detection limit of each element. The values were expressed as mean ± standard deviation, mean (95% confidential interval), and number (proportion) for descriptive statistics. For the variables of the minerals that had a right-skewed distribution, the values were analyzed using logarithmically transformed data. Independent sample t test was used for the comparison of mean values of age, waist circumference, body weight, height, BMI, and minerals between the fibromyalgia group and the reference group. Chi-square test was used for the comparison of proportion of current smokers and current drinkers between the groups. To study the relationship between minerals and fibromyalgia, principal component analysis was used to reduce the dimensionality of the mineral data. The factors with eigenvalues > 1.0 were retained in the analysis. A varimax orthogonal rotation was applied to identify any distinct latent factors and to facilitate interpretation. The differences of factors between the groups were analyzed using t test. For multivariate analysis, each factor entered in the logistic regression model (Model 1), and age was adjusted (Model 2). In the final model (Model 3), waist circumference, alcohol and smoking were also adjusted. For multivariate analysis, age was categorized into three groups (< 40, 40-49, and ≥ 50 yr), and waist circumference was divided into central obesity (≥ 80 cm) or nonobesity. A P value < 0.05 was considered statistically significant. SPSS version 18 (SPSS, Chicago, IL, USA) was used for all statistical analyses.
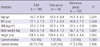
Forty-four women with fibromyalgia were enrolled in the case group. Among the 683 women who had their hair tested, 205 had appropriate anthropometry and historical data. Eighty-three women were excluded from the reference group. The mean age of the all subjects was 43.7 ± 8 yr and there was no significant difference between the cases and the reference group. The values of the anthropometric measures and proportions of current smokers and drinkers were not statistically different between the groups (Table 1). The results of hair minerals analysis revealed statistical differences in the levels of calcium (1,093 µg/g for reference group, 775 µg/g for fibromyalgia group), magnesium (72 µg/g for reference group, 52 µg/g for fibromyalgia group), copper (40.2 µg/g for reference group, 28.3 µg/g for fibromyalgia group), iron (7.1 µg/g for reference group, 5.9 µg/g for fibromyalgia group), and manganese (190 ng/g for reference group, 140 ng/g for fibromyalgia group) (Table 2).
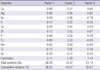
Factor analysis was used to evaluate the relationship among significant hair minerals. Hair mineral elements were reduced to three factors by principal component analysis. Factor loadings after varimax rotation are shown in Table 3. Calcium, magnesium, iron, and manganese were loaded at Factor 1. Factor 2 was characterized by sodium, potassium, and chromium. Zinc and selenium comprised Factor 3. The mean of Factor 1 for the fibromyalgia group was significantly lower than that for the reference group by t test (-0.374 for fibromyalgia group, 0.135 for reference group, P = 0.004) (Table 4). According to the result of multivariate analysis, the mean of Factor 1 for the fibromyalgia group was lower than that for the reference group even after adjustment of multiple potential confounders (Table 5).
In this study, hair minerals in fibromyalgia patients were analyzed and compared with those in a reference group of age- and BMI-matched female patients who did not have fibromyalgia. Univariate analyses revealed differences between the two groups in several elements such as calcium, magnesium, copper, iron, and manganese. Upon principal component analysis, calcium, magnesium, iron, and manganese were the components of a single factor, which was significantly lower in the fibromyalgia group even after adjustment of multiple potential confounders.
Several mechanisms have been suggested to explain the pathophysiology of fibromyalgia. Alterations in muscle are an important aspect of fibromyalgia (19, 20). Another hypothesis involves impaired functioning of the hypothalamic-pituitary-adrenal axis (21). Sleep pattern change is also frequently observed in fibromyalgia patients (22). A greater understanding of cellular mechanisms is needed to explain these hypotheses. Therefore, it is important to detect the alteration of trace minerals in fibromyalgia patients.
A few studies have probed the characteristics of elements in fibromyalgia patients. Rosborg et al. (13) investigated the minerals in the serum and urine of fibromyalgia patients, but failed to find significant characteristics. The levels of trace minerals in fibromyalgia patients are controversial. In one study, serum selenium, magnesium, zinc, vitamin B1, B2, vitamins A and E were analyzed; magnesium in leukocytes was elevated, but no significant differences among the other minerals were found (12).
Hypocalcemia can cause extensive spasm of skeletal muscle, cramps and tetany (23). However, most patients with muscle pain including fibromyalgia have a normal range of serum calcium. It is also true that even if calcium concentration in the blood is normal, the intracellular calcium level can be different. Magaldi et al. (24) investigated the difference of intracellular calcium level between fibromyalgia cases and controls. They found that in fibromyalgia patients the intracellular calcium concentration is significantly reduced in comparison to that of healthy controls, which may be potentially responsible for muscular hypertonus (24). In another study, lower bone mineral density was noted in fibromyalgia patients than in controls, perhaps an indirect indication of impaired calcium metabolism in fibromyalgia (25).
Hypomagnesemia causes generalized alterations in neuromuscular function and is related with muscle weakness and cramps (23). Hypomagnesemia can cause impaired synthesis of 1, 25(OH)2D; vitamin D deficiency can also cause hypocalcemia (23). Some studies have provided evidence of lowered magnesium status in fibromyalgia and other conditions. In one study, fibromyalgia cases and controls were investigated for trace minerals (14). Zinc and magnesium in fibromyalgia patients was lower than those in controls, but selenium did not differ appreciably. Low magnesium concentration in erythrocytes was reported in chronic sleep deprivation (26). Magnesium deficit was noted in some individuals with chronic fatigue (27). A double-blind study had been carried out to test the efficacy and the safety of malic acid and magnesium supplementation for treatment of fibromyalgia (28). To date, magensium supplementation has been applied to non-pharmacological therapy (29).
A study investigated malondialdehyde and superoxide disumutase in 85 female fibromyalgia patients and controls; and reported that oxidant/antioxidant balances are changed in fibromyalgia (10). Another study investigated 30 female fibromyalgia patients in comparison with controls. This study evaluated superoxide dismutase, xanthine oxidase, adenosine deaminase enzyme activity, thiobarbituric acid reactive substances and nitric oxide levels; high level of thiobarbituric acid reactive substances and low level of nitric oxide were detected in fibromyalgia (11). These collective data imply that the scavenging capacity of reactive oxygen species is reduced in fibromyalgia.
In humans, three forms of superoxide dismutase are present. They contain some minerals such as copper, zinc and manganese. Manganese, especially, is an essential element comprising superoxide dismutase 2 that functions in antioxidant defense in mitochondria (30). Considering that oxidant/antioxidant balances were altered in fibromyalgia, an insufficiency of manganese might be related with pathophysiology of fibromyalgia.
There are some limitations in our study. First, since the study design was cross-sectional, causality cannot be explained. To ascertain causality, a longitudinal study would be required. Second, the controls in our study were patients with other problems. However, factors associated with fibromyalgia were excluded according to the criteria in our study. Third, the mineral assay was done only in hair tissue. Minerals in serum and urine were not analyzed, so the consistency with prior studies could not be confirmed. However, the hair mineral assay is a good method to explore the mineral status at the cellular level.
In conclusion, the concentrations of calcium, magnesium, iron, and manganese in the hair of female patients with fibromyalgia are lower than of controls, even after adjustment of potential confounders. Whether supplementation with minerals would have an impact on the progress of fibromyalgia should be considered in more detail. Also, factors influencing the pain in fibromyalgia patients should be further evaluated.
Figures and Tables
ACKNOWLEDGMENTS
This research is supported by the Ubiquitous Computing and Network (UCN) Project, the Ministry of Knowledge and Economy (MKE) and Economy Frontier R&D Program in Korea, by the Korea Breast Cancer Foundation, and we thank the TEI (Trace Element Inc.) Korea for the hair mineral analysis for this study.
AUTHOR SUMMARY
Women with Fibromyalgia Have Lower Levels of Calcium, Magnesium, Iron and Manganese in Hair Mineral Analysis
Young-Sang Kim, Kwang-Min Kim, Duck-Joo Lee, Bom-Taeck Kim, Sat-Byul Park, Doo-Yeoun Cho, Chang-Hee Suh, Hyoun-Ah Kim, Rae-Woong Park and Nam-Seok Joo
We compared the hair mineral between fibromyalgia patients healthy control group by using factor analysis. Fibromyalgia patients displayed a significantly lower level of calcium, magnesium, copper, iron and manganese, independent of potential confounders.
References
1. White KP, Speechley M, Harth M, Ostbye T. The London Fibromyalgia Epidemiology Study: comparing the demographic and clinical characteristics in 100 random community cases of fibromyalgia versus controls. J Rheumatol. 1999. 26:1577–1585.
2. Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995. 38:19–28.
3. Simms RW. Fibromyalgia is not a muscle disorder. Am J Med Sci. 1998. 315:346–350.
4. Yunus MB. Towards a model of pathophysiology of fibromyalgia: aberrant central pain mechanisms with peripheral modulation. J Rheumatol. 1992. 19:846–850.
5. Yunus MB, Dailey JW, Aldag JC, Masi AT, Jobe PC. Plasma tryptophan and other amino acids in primary fibromyalgia: a controlled study. J Rheumatol. 1992. 19:90–94.
6. Hrycaj P, Stratz T, Müller W. Platelet 3H-imipramine uptake receptor density and serum serotonin levels in patients with fibromyalgia/fibrositis syndrome. J Rheumatol. 1993. 20:1986–1988.
7. Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, Gracely RH, Clauw DJ. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003. 48:2916–2922.
8. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003. 163:2433–2445.
9. Wolfe F, Anderson J, Harkness D, Bennett RM, Caro XJ, Goldenberg DL, Russell IJ, Yunus MB. Health status and disease severity in fibromyalgia: results of a six-center longitudinal study. Arthritis Rheum. 1997. 40:1571–1579.
10. Bagis S, Tamer L, Sahin G, Bilgin R, Guler H, Ercan B, Erdogan C. Free radicals and antioxidants in primary fibromyalgia: an oxidative stress disorder? Rheumatol Int. 2005. 25:188–190.
11. Ozgocmen S, Ozyurt H, Sogut S, Akyol O, Ardicoglu O, Yildizhan H. Antioxidant status, lipid peroxidation and nitric oxide in fibromyalgia: etiologic and therapeutic concerns. Rheumatol Int. 2006. 26:598–603.
12. Eisinger J, Plantamura A, Marie PA, Ayavou T. Selenium and magnesium status in fibromyalgia. Magnes Res. 1994. 7:285–288.
13. Rosborg I, Hyllén E, Lidbeck J, Nihlgård B, Gerhardsson L. Trace element pattern in patients with fibromyalgia. Sci Total Environ. 2007. 385:20–27.
14. Sendur OF, Tastaban E, Turan Y, Ulman C. The relationship between serum trace element levels and clinical parameters in patients with fibromyalgia. Rheumatol Int. 2008. 28:1117–1121.
15. Joo NS, Kim SM, Jung YS, Kim KM. Hair iron and other minerals' level in breast cancer patients. Biol Trace Elem Res. 2009. 129:28–35.
16. Park SB, Choi SW, Nam AY. Hair tissue mineral analysis and metabolic syndrome. Biol Trace Elem Res. 2009. 130:218–228.
17. Ng SY. Hair calcium and magnesium levels in patients with fibromyalgia: a case center study. J Manipulative Physiol Ther. 1999. 22:586–593.
18. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, Mccain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990. 33:160–172.
19. Bengtsson A, Henriksson KG, Larsson J. Muscle biopsy in primary fibromyalgia. Light-microscopical and histochemical findings. Scand J Rheumatol. 1986. 15:1–6.
20. Jacobsen S, Bartels EM, Danneskiold-Samsoe B. Single cell morphology of muscle in patients with chronic muscle pain. Scand J Rheumatol. 1991. 20:336–343.
21. Crofford LJ, Pillemer SR, Kalogeras KT, Cash JM, Michelson D, Kling MA, Sternberg EM, Gold PW, Chrousos GP, Wilder RL. Hypothalamic-pituitary-adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheum. 1994. 37:1583–1592.
22. Wolfe F. Fibromyalgia: the clinical syndrome. Rheum Dis Clin North Am. 1989. 15:1–18.
23. Jameson JL. Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Endocrinology and metabolism. Harrison's principles of internal medicine. 2008. 17th ed. New York: McGraw-Hill Medical;2372–2373.
24. Magaldi M, Moltoni L, Biasi G, Marcolongo R. Role of intracellular calcium ions in the physiopathology of fibromyalgia syndrome. Boll Soc Ital Biol Sper. 2000. 76:1–4.
25. Dessein PH, Stanwix AE. Why would fibromyalgia patients have osteoporosis? J Rheumatol. 2000. 27:1816–1817.
26. Tanabe K, Osada N, Suzuki N, Nakayama M, Yokoyama Y, Yamamoto A, Oya M, Murabayashi T, Yamamoto M, Omiya K, Itoh H, Murayama M. Erythrocyte magnesium and prostaglandin dynamics in chronic sleep deprivation. Clin Cardiol. 1997. 20:265–268.
27. Moorkens G, Manuel-y-Keenoy B, Vertommen J, Meludu S, Noe M, De Leeuw I. Magnesium deficit in a sample of the Belgian population presenting with chronic fatigue. Magnes Res. 1997. 10:329–337.
28. Russell IJ, Michalek JE, Flechas JD, Abraham GE. Treatment of fibromyalgia syndrome with Super Malic: a randomized, double blind, placebo controlled, crossover pilot study. J Rheumatol. 1995. 22:953–958.
29. Porter NS, Jason LA, Boulton A, Bothne N, Coleman B. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. 2010. 16:235–249.
30. Bendich A. Antioxidant micronutrients and immune responses. Ann N Y Acad Sci. 1990. 587:168–180.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download