Abstract
We developed and validated a novel Korean prostate cancer risk calculator (KPCRC) for predicting the probability of a positive initial prostate biopsy in a Korean population. Data were collected from 602 Koreans who underwent initial prostate biopsies due to an increased level of prostate-specific antigen (PSA), a palpable nodule upon digital rectal examination (DRE), or a hypoechoic lesion upon transrectal ultrasound (TRUS). The clinical and laboratory variables were analyzed by simple and multiple logistic regression analysis. The area under the receiver operating characteristic curve (AUC) was computed to compare its performance to PSA testing alone. Prostate cancer was detected in 172 (28.6%) men. Independent predictors included age, DRE findings, PSA level, and prostate transitional zone volume. We developed the KPCRC using these variables. The AUC for the selected model was 0.91, and that of PSA testing alone was 0.83 (P < 0.001). The AUC for the selected model with an additional dataset was 0.79, and that of PSA testing alone was 0.73 (P = 0.004). The calculator is available on the website: http://pcrc.korea.ac.kr. The KPCRC improved the performance of PSA testing alone in predicting the risk of prostate cancer in a Korean population. This calculator would be a practical tool for physicians and patients.
Prostate cancer is the most common cancer and the second most common cause of cancer related death in the United States (1). Even though the incidence of prostate cancer in Korea is not as high as in the United States, it is swiftly increasing to reach the 5th most common malignancy in Korean men (2). In 2005, 3,487 new cases and 900 deaths from prostate cancer in Korea were recorded (3). According to this article, the annual percent change of prostate cancer is 12.6%, which was calculated from the prostate cancer incidence 8.4 (per 100,000) in 1999 to 14.7 in 2005. Another paper reported 4,425 new cases and 1,004 deaths in 2006 and 5,292 new cases and 1,107 deaths in 2007 from prostate cancer in Korea (2). The age-standardized mortality rates have also continued to increase; 4.3 (per 100,000) in 2005, 4.5 per 2006 and 4.6 in 2007 (2, 3). In this situation, increasing the information about prostate cancer provided by mass media or lower urinary tract symptoms motivates patients to see the physicians and get some screening tests. After an abnormal screening test patients want to seek the most objective information about the probability of prostate cancer diagnosis. Similarly physicians may wish to predict the probability of positive biopsy before biopsy is recommended (1).
Since the introduction of prostate-specific antigen (PSA) testing in 1987, serum PSA has become a useful tool in screening for prostate cancer (4). However, it is still difficult to differentially diagnose prostate cancer from benign prostatic disease because PSA levels depend on age, prostate size, and the inflammatory state of the prostate (5, 6). In addition to total PSA, there are other clinical factors that improve the detection rate of prostate cancer, such as age, digital rectal examination (DRE) findings, transrectal ultrasound (TRUS) findings, PSA density (PSAD), PSA velocity, PSAD of transition zone volume (PSADT), percent of free PSA (% free PSA), and age-specific PSA (7-9). However, aside from % free PSA, the value of the other methods remains a subject of considerable debate (10).
The tools that could precisely predict the presence of cancer prior to biopsy would significantly reduce the number of unnecessary prostate biopsies among males with elevated serum PSA levels, for whom it is currently difficult to determine whether a prostate biopsy is indicated (10). In addition, such tools could provide patients with their probability of prostate cancer prior to biopsy. Recently, some nomograms using clinical, laboratory, and ultrasound parameters have been reported, and racial differences have been recognized as an important parameter that influences prostate cancer incidence (11-13). Therefore, the nomograms based on Western populations might not be appropriate for use in the Korean population due to differences in risk factors for prostate cancer between Asian and Western populations. Several nomograms for the Japanese population have been reported, but no prediction model for the Korean population has been published, except for a report in one Korean journal (10, 14, 15). Therefore, we decided to create a multiple logistic regression model that assigns a probability of detecting prostate cancer upon initial TRUS-guided biopsy in a Korean population. In order to determine its clinical utility at the selected probability cutoffs, we also validated this model internally and externally with our dataset and an additional dataset from the affiliated hospital, respectively. This model can be used as a robust tool to improve the prediction of prostate cancer.
Between January 2004 and December 2008, a total of 670 cases of TRUS-guided ten-core biopsy were performed because of suspicion of prostate cancer-increased level of PSA, a palpable nodule upon DRE, or a hypoechoic lesion upon TRUS. We excluded 15 cases with incomplete medical records, 5 cases with a PSA level of 1,000 ng/mL or more, and 48 cases of two or more repeated biopsies. Data were collected retrospectively and uniformly from 602 cases of initial TRUS-guided biopsy.
We chose several factors to evaluate the following important predictors for a positive prostate biopsy: age, DRE findings, total PSA level, free PSA level, % free PSA, TRUS findings, prostate volume, prostate transitional zone volume, PSAD, and PSADT. DRE was classified as normal or abnormal (any prostatic nodule or induration). Serum PSA and free PSA tests were performed using the automated chemiluminescent microparticle immunoassay analyzer Architect i2000 (Abbott Diagnostic Laboratories, Abbott Park, IL, USA). TRUS findings were classified as normal or abnormal (any presence of hypoechoic lesion). The prostate was measured in three dimensions, and its volume was estimated using a modification of the prolate ellipsoid formula and recorded in cm3 (0.523 [length (cm) × width (cm) × height (cm)]) by TRUS (10). After identifying the transitional zone by TRUS, the volume was measured by the same method described above. PSAD and PSADT were calculated by dividing the serum PSA level by the calculated prostate volume and calculated transitional zone volume, respectively. A member of a urology team performed a DRE on all patients before the TRUS.
The informed consents were obtained from all of the subjects before the intervention. Disinfecting rectal cleaning with 10% povidone iodine and prophylactic antibiotics was performed prior to the TRUS-guided biopsy. All biopsies were performed with an automatic 18-gauge biopsy needle (Bard Urological Division, Covington, GA, USA) in conjunction with a Hawk 2102EXL medical ultrasound scanner (BK Medical A/S, Mileparken 34, DK-2730 Herlev, Denmark). A minimum of ten cores, including samples from each case, with additional cores when indicated were taken from suspicious areas. The biopsy specimens were examined for the presence of cancer and were categorized using the Gleason score by a pathologist.
The significance of each factor was assessed by simple logistic regression analysis. Multiple logistic regression analysis with a backward variable selection procedure was used to determine which factors were independent predictors of prostate cancer in the model-building set. PSA level, prostate volume, and prostate transitional zone volume were log-transformed prior to analysis. A prediction equation (Park's prediction equation) for positive biopsy was developed based on the final logistic regression model. Based on the receiver operating characteristic (ROC) curve, we selected the cut-off value for the predicted probability of prostate cancer, providing a high sensitivity and concomitantly reducing the total number of unnecessary biopsies. We compared the performance of our model with that of a model using the PSA level alone by calculating the area under the receiver operating characteristic curve (AUC) and assessing its statistical significance with the method proposed by Hanley and McNeil (16). We also compared the performance of these 2 models by the same method with an additional dataset of 324 patients from an affiliated hospital. Data are expressed as either the mean±standard deviation (SD), median [inter-quartile range], or percentage (%) of cases. All statistical outcomes are presented as the odds ratio (OR) and the 95% confidence interval (95% CI) based on a two-sided test using the SAS statistical package (Version 9.1; SAS Institute, Cary, NC, USA). We regarded a P value < 0.05 as statistically significant.
The characteristics of the study population are shown in Table 1. Adenocarcinoma of the prostate was detected upon biopsy in 28.6% of men (172 of 602 cases). The remaining 430 cases included 393 cases of benign prostatic hyperplasia, 34 of prostatitis, and 3 of high-grade prostatic intraepithelial neoplasia. Among the 172 cases with a positive biopsy result, the assigned Gleason score was 2-4 in 5 cases (2.9%), 6 in 47 cases (27%), 7 in 35 cases (20%), and 8-10 in 85 cases (50%). In 123 men with a serum PSA level or 4.0 ng/mL or less, a positive biopsy was detected in 10 cases (8.1%), representing 5.8% of all prostate cancer cases detected in the current study (10 of 172 cases). Among these 10 cases, the assigned Gleason score was 6 in 5 cases (50%), 7 in 2 cases (20%), and 8-9 in 3 cases (30%).
In the simple logistic regression analysis, all of the variables listed above were statistically significant predictors of prostate cancer upon needle biopsy (all P < 0.001) (Table 2). In the multiple logistic regression analysis with a backward variable selection procedure, the significant predictors of a positive prostate biopsy for all patients were age, DRE findings, PSA level, and prostate transitional zone volume (Table 2). The following Park's prediction equation was developed utilizing the four independent risk factors for predicting a positive result of prostate cancer:
where the T-volume represents the prostate transitional zone volume. As for continuous variables such as age, PSA, and prostate transitional zone volume, the value itself was put into the equation. The age range was 36-89 yr, the PSA range was 0.45-893, and the range of prostate transitional zone volumes was 3-120. As for the categorical variable of DRE finding, 0 was used in the equation when normal, and 1 was used when abnormal. For example, a 60-yr old man with a PSA of 8 ng/mL had an abnormal DRE, and a prostate transitional zone volume of 15 cm3. According to this equation, his risk of a positive biopsy would be 42%, not the 25% risk commonly quoted by a PSA level in the intermediate range (4-10 ng/mL) (14). Using this equation, the possibility for a positive biopsy ranges from 0.1% to 68.9% for patients with a PSA level 4 ng/mL or less.
When 0.2642 was used as the cutoff value in the equation, the positive predictive value, the negative predictive value, the sensitivity, and the specificity were 71%, 92%, 81%, and 87%, respectively. This cutoff value would prevent 87% of unnecessary negative biopsies, but 19% of prostate cancer cases would be overlooked. When the cutoff value of 0.1 was used, the positive predictive value, the negative predictive value, the sensitivity, and the specificity were 47%, 95%, 93%, and 59%, respectively. When 4 ng/mL was used as the cutoff value of the PSA level, the positive predictive value, the negative predictive value, the sensitivity, and the specificity were 34%, 92%, 94%, and 26%, respectively. The ROC curve demonstrated an AUC of 0.91 for this model (95% CI, 0.88-0.93) and 0.83 for the prediction based on PSA alone (95% CI, 0.79-0.87), yielding significantly different values (P < 0.001) (Fig. 1A).
The accuracy of the model was determined using the validation set. When 0.1 was used as the cutoff value of the equation, the positive predictive value, the negative predictive value, the sensitivity, and the specificity were 45%, 88%, 90%, and 41%, respectively. When 4 ng/mL was used as the cutoff value of the PSA level, the positive predictive value, the negative predictive value, the sensitivity, and the specificity were 39%, 86%, 98%, and 6%, respectively. The ROC curve was used to evaluate the accuracy of the predicted probability from the model, as compared with the prediction based on total PSA alone (Fig. 1B). The AUC was 0.79 for this model (95% CI, 0.74-0.85) and 0.73 for the prediction based on PSA alone (95% CI, 0.68-0.79), which represents a significant difference, indicating the superiority of our model in differentiating between benign and malignant disease (P = 0.004). The receiver operating characteristic curve evaluated the accuracy of the prediction model and the other predictors for the 169 patients with PSA level 4-10 ng/mL (Table 3). The accuracy of the predicted probability of our model was 72%. It was higher than those of other predictors such as PSA, % free PSA, PSAD, and PSADT, but the differences did not reach statistical significance. Using this equation, we developed a novel Korean prostate cancer risk calculator. The calculator is available on the following website: http://dna.korea.ac.kr.
We evaluated ten risk factors associated with prostate cancer work up, which were commonly available to urologists in Korea. These risk factors included the basic clinical information (age, DRE findings), PSA test results, TRUS findings, and their derivatives (% free PSA, prostate volume, prostate transitional zone volume, PSAD, and PSADT). Although TRUS is performed for prostate biopsy, previous studies have predicted biopsy results based only on clinical and laboratory findings without TRUS (1, 17, 18), or evaluated TRUS findings or prostate volume without prostate transitional zone volume, PSAD, and PSADT (14, 19). The present study is the first assessment of these factors.
Karakiewicz et al. (1) have shown that the combination of age, DRE findings, PSA and % free PSA yields an AUC of 77% in prediction of prostate cancer on needle biopsy for the patients whose PSA level was not greater than 50 ng/mL. Most urologists in Korea perform PSA testing and TRUS due to the lower medical costs. On the contrary, free PSA is not covered by the National Health Insurance in Korea when total PSA level is below 4 ng/mL. As a result, free PSA is not so widely available for the new patients whose total PSA level is uncertain. Therefore, TRUS variables would be more practical than % free PSA for the prostate cancer predictor in Korea. Suzuki et al. developed and validated an initial biopsy nomogram predicting prostate cancer in Japanese patients which yielded a predictive accuracy of about 80% (14). They used age, DRE findings, PSA, % free PSA and prostate volume measured by TRUS as significant predictors. The accuracy of nomogram in this study is similar to our results although the numbers of the variables are more than four, which means that the prediction performance is not so efficient.
Including these previous articles mentioned above, many studies have investigated the predictive model for prostate cancer using age, abnormal DRE findings, and elevated total PSA level (1, 14, 15, 18). However, the efficacy of prostate transitional zone volume in the predictive model has not been mentioned yet. It has been reported that a larger volume of the prostate may predict a lower possibility of prostate cancer (14). Therefore, large prostate volume may be a protective factor for detecting prostate cancer. Considering OR of prostate and prostate transitional zone volume in simple and multiple logistic regression in this study and that benign prostatic hyperplasia is almost exclusively the result of hyperplasia of the transition zone (20), the volume of the prostate transitional zone can be potentially a more protective factor. There is a report supporting the hypothesis that PSADT is more accurate in predicting a positive biopsy than PSAD for patients with PSA levels between 4.1 ng/mL and 10.0 ng/mL (7). The issue has been also investigated in Korea. Kang and colleagues assessed that PSADT might be more useful than % free PSA or PSAD for patients with the same PSA levels (21).
According to the results of the multiple logistic regression analysis, the independent factors associated with a positive biopsy were more advanced age, abnormal DRE findings, elevated total PSA level, and smaller prostate transitional zone volume. We used these factors to develop a predictive model for detecting prostate cancer in order to reduce the number of unnecessary prostate biopsies. Using a cancer probability cutoff level of 10%, we predicted a reduction in the number of unnecessary biopsies by 59%, while maintaining a sensitivity of 93%. With the dataset for external validation, if we decide that only those patients with 10% predicted probability of prostate cancer will undergo a biopsy, then our model captures 90% of all prostate cancer cases (sensitivity), while sparing 41% of patients without prostate cancer from undergoing an unnecessary procedure (specificity). On the other hand, with the cutoff value of 4 ng/mL PSA, 6% of unnecessary negative biopsies would be prevented, but 2% of prostate cancer cases would be overlooked. Considering the potential complication with TRUS-guided biopsy, efforts should be directed toward avoiding unnecessary biopsies as well as preventing failure to diagnose cancer. In addition, using this equation when the significant predictors are present, the possibility for a positive biopsy ranges up to about 70% in case of a PSA level 4 ng/mL or less. From this point of view, PSA alone may not be a proper tool for deciding whether the biopsy should be performed as indicated by other investigators (9, 17).
Our grade distribution had a high proportion of Gleason score 7 or higher. This may be due to the fact that we included patients with PSA levels up to 900 ng/mL. However, the detection rates of previous studies were between 20% and 40%, which was not so different from 28.6% of our result (10-15). Moreover, most of these studies did not describe the Gleason score distribution, which made the direct comparison impossible. Song et al. (22) reported that, unlike western population, proportion of patients with Gleason scores 7 or higher was more than half of each subgroup throughout the clinical stages and PSA ranges and concluded that prostate cancers arising in Korean men exhibit poor differentiation regardless of the initial serum PSA level or clinical stage at presentation. Our grade distribution might be the reflection of the racial difference in prostate cancer characteristics.
The limitation of Park's prediction equation is that it is not simple enough for patients to use. For this reason, we developed a novel KPCRC and made it available on the web. Now that internet access is easily available throughout Korea, this web-based calculator can be used by both physicians and patients without any difficulty. For the patients with very high PSA level, we decided the range of PSA levels up to 900 ng/mL. With the calculator on the website, they can calculate their results at home. On the contrary, this model may not be directly applicable to Korean men living outside of Asia. However, given that nomograms developed in Western countries cannot be directly applied to the Asian male population (14), the KPCRC can be used to precisely predict the probability of a positive prostate biopsy for the selected population. In the present study, we showed that the KPCRC improved the performance of PSA testing alone in predicting the risk of prostate cancer in a Korean population. This is the first study to address a web-based prediction model using Korean population data. The model will provide physicians and patients in Korea with information that can be used in deciding whether to undergo prostate biopsy.
Figures and Tables
Fig. 1
Receiver operating characteristics curve for our model and prostate specific antigen (A) in 602 patients who underwent initial prostate biopsies (P < 0.001), (B) in 324 patients from additional dataset for the external validation (P = 0.004).

Table 2
The simple and multiple logistic regression model analyzing the predictors of prostate cancer detection upon initial prostate biopsy

*Backward variable selection procedure was applied. OR, Odds ratio; CI, confidence interval; DRE, digital rectal examination; PSA, prostate-specific antigen; TRUS, transrectal ultrasound; P-volume, prostate volume; T-volume, prostate transitional zone volume; PSAD, PSA density; PSADT, PSAD of transition zone volume.
AUTHOR SUMMARY
Initial Biopsy Outcome Prediction in Korean Patients-Comparison of a Novel Web-based Korean Prostate Cancer Risk Calculator versus Prostate-specific Antigen Testing
Jae Young Park, Sungroh Yoon, Man Sik Park, Dae-Yeon Cho, Hong-Seok Park, Du Geon Moon, and Duck Ki Yoon
We developed and validated a novel Korean prostate cancer risk calculator (KPCRC) for predicting the probability of a positive initial prostate biopsy in a Korean population. Data were collected from 602 Koreans who underwent initial prostate biopsies. The clinical and laboratory variables were analyzed by simple and multiple logistic regression analysis. The area under the receiver operating characteristic curve (AUC) was computed to compare its performance to PSA testing alone. Prostate cancer was detected in 172 (28.6%) men. Independent predictors included age, DRE findings, PSA level, and prostate transitional zone volume. The AUC for the selected model was 0.91, and that of PSA testing alone was 0.83. The AUC for the selected model with an additional dataset was 0.79, and that of PSA testing alone was 0.73. The KPCRC improved the performance of PSA testing alone in predicting the risk of prostate cancer in a Korean population.
References
1. Karakiewicz PI, Benayoun S, Kattan MW, Perrotte P, Valiquette LUC, Scardino PT, Cagiannos I, Heinzer H, Tanguay S, Aprikian AG, Huland H, Graefen M. Development and validation of a nomogram predicting the outcome of prostate biopsy based on patient age, digital rectal examination and serum prostate specific antigen. J Urol. 2005. 173:1930–1934.
2. Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010. 25:1113–1121.
3. Jung KW, Won YJ, Park S, Kong HJ, Sung J, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009. 24:995–1003.
4. Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991. 145:907–923.
5. el-Galley RE, Petros JA, Sanders WH, Keane TE, Galloway NT, Cooner WH, Graham SD Jr. Normal range prostate-specific antigen versus age-specific prostate-specific antigen in screening prostate adenocarcinoma. Urology. 1995. 46:200–204.
6. Nadler RB, Humphrey PA, Smith DS, Catalona WJ, Ratliff TL. Effect of inflammation and benign prostatic hyperplasia on elevated serum prostate specific antigen levels. J Urol. 1995. 154:407–413.
7. Kalish J, Cooner WH, Graham SD Jr. Serum PSA adjusted for volume of transition zone (PSAT) is more accurate than PSA adjusted for total gland volume (PSAD) in detecting adenocarcinoma of the prostate. Urology. 1994. 43:601–606.
8. Gohji K, Nomi M, Egawa S, Morisue K, Takenaka A, Okamoto M, Ohori M, Fujii A. Detection of prostate carcinoma using prostate specific antigen, its density, and the density of the transition zone in Japanese men with intermediate serum prostate specific antigen concentrations. Cancer. 1997. 79:1969–1976.
9. Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, Patel A, Richie JP, deKernion JB, Walsh PC, Scardino PT, Lange PH, Subong EN, Parson RE, Gasior GH, Loveland KG, Southwick PC. Use of the percentage of free prostate-apecific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998. 279:1542–1547.
10. Kawamura K, Suzuki H, Kamiya N, Imamoto T, Yano M, Miura J, Shimbo M, Suzuki N, Nakatsu H, Ichikawa T. Development of a new nomogram for predicting the probability of a positive initial prostate biopsy in Japanese patients with serum PSA levels less than 10 ng/mL. Int J Urol. 2008. 15:598–603.
11. Garzotto M, Hudson RG, Peters L, Hsieh YC, Barrera E, Mori M, Beer TM, Klein T. Predictive modeling for the presence of prostate carcinoma using clinical, laboratory, and ultrasound parameters in patients with prostate specific antigen levels < or = 10 ng/mL. Cancer. 2003. 98:1417–1422.
12. Yanke BV, Carver BS, Bianco FJ Jr, Simoneaux WJ, Venable DD, Powell IJ, Eastham JA. African-American race is a predictor of prostate cancer detection: incorporation into a pre-biopsy nomogram. BJU Int. 2006. 98:783–787.
13. Nam RK, Toi A, Klotz LH, Trachtenberg J, Jewett MA, Appu S, Loblaw DA, Sugar L, Narod SA, Kattan MW. Assessing individual risk for prostate cancer. J Clin Oncol. 2007. 25:3582–3588.
14. Suzuki H, Komiya A, Kamiya N, Imamoto T, Kawamura K, Miura J, Suzuki N, Nakatsu H, Hata A, Ichikawa T. Development of a nomogram to predict probability of positive initial prostate biopsy among Japanese patients. Urology. 2006. 67:131–136.
15. Cho TW, Kim SH, Park D. Development of statistical model for predicting prostate cancer in patients requiring prostate biopsy. Korean J Urol. 2004. 45:1014–1020.
16. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983. 148:839–843.
17. Carlson GD, Calvanese CB, Partin AW. An algorithm combining age, total prostate-specific antigen (PSA), and percent free PSA to predict prostate cancer: results on 4298 cases. Urology. 1998. 52:455–461.
18. Chun FK, Briganti A, Graefen M, Montorsi F, Porter C, Scattoni V, Gallina A, Walz J, Haese A, Steuber T, Erbersdobler A, Schlomm T, Ahyai SA, Currlin E, Valiquette L, Heinzer H, Rigatti P, Huland H, Karakiewicz PI. Development and external validation of an extended 10-core biopsy nomogram. Eur Urol. 2007. 52:436–445.
19. Kawakami S, Numao N, Okubo Y, Koga F, Yamamoto S, Saito K, Fujii Y, Yonese J, Masuda H, Kihara K, Fukui I. Development, validation, and head-to-head comparison of logistic regression-based nomograms and artificial neural network models predicting prostate cancer on initial extended biopsy. Eur Urol. 2008. 54:601–611.
20. Zlotta AR, Djavan B, Marberger M, Schulman CC. Prostate specific antigen density of the transition zone: a new effective parameter for prostate cancer prediction. J Urol. 1997. 157:1315–1321.
21. Kang SH, Bae JH, Park HS, Yoon DK, Moon DG, Kim JJ, Cheon J. Prostate-specific antigen adjusted for the transition zone volume as a second screening test: a prospective study of 248 cases. Int J Urol. 2006. 13:910–914.
22. Song C, Ro JY, Lee MS, Hong SJ, Chung BH, Choi HY, Lee SE, Lee E, Kim CS, Ahn H. Prostate cancer in Korean men exhibits poor differentiation and is adversely related to prognosis after radical prostatectomy. Urology. 2006. 68:820–824.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


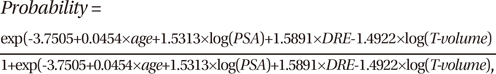

 XML Download
XML Download