Abstract
A seventeen-year-old female patient was admitted with sudden-onset of headache and vomiting. Brain magnetic resonance imaging demonstrated a heterogeneously enhancing tumour in the left lateral ventricle. The tumour was removed and confirmed as a central neurocytoma (CN). For the residual tumour in the left lateral ventricle, gamma knife stereotactic radiosurgery was done at fifteen months after the initial surgery. Tumour recurred in the 4th ventricle at 5 yr after initial surgery. The tumour was removed and proved as a CN. In vitro primary culture was done with both tumours obtained from the left lateral ventricle and the 4th ventricle, respectively. Nestin, a neuronal stem cell marker was expressed in reverse Transcriptase-Polymerase Chain Reaction of both tumors. Both tumours showed different morphology and phenotypes of neuron and glia depending on the culture condition. When cultured in insulin, transferrin selenium and fibronectin media with basic fibroblast growth factors, tumour cells showed neuronal morphology and phenotypes. When cultured in the Dulbeco's Modified Essential Media with 20% fetal bovine serum, tumors cells showed glial morphology and phenotypes. It is suggested that CN has the characteristics of neuronal stem cells and potential to differentiate into mature neuron and glial cells depending on the environmental cue.
Stem cells are defined as self-renewing, multipotent cells that have the capacity of multilineage differentiation (1). The multipotential, self-renewing neuronal stems cells have been identified in adult mammalian brain in the past decade (2). Two germinal regions are known to persist in the adult mammalian brain possessing the neuronal stem cells: the subventricular zone (SVZ) of the lateral ventricle, and the subgranular zone (SGZ) of the hippocampal formation (3). It was also reported that infusion of epidermal growth factor (EGF) or fibroblastic growth factor (FGF) into the brain ventricles dramatically expanded the SVZ cell population (4).
A peculiar brain tumour called "central neurocytoma (CN)" has been reported to develop in the SVZ area of the lateral ventricle (5). The CN is known as a well-differentiated neuronal tumour characterized by having synaptic structures, clear and dense-cored vesicles, and parallel microtubular structures. However, several reports have issued that CN had the capacity to differentiate into both neurons and glia in in vitro culture environment (6-10).
The authors reported a case with an experience of In vitro primary culture of CN, which showed different morphology and phenotypes of neuron and glia depending on the different culture condition.
A seventeen-year-old female patient was admitted presenting sudden-onset of headache and vomiting. No focal neurological deficit except papilledema was found. Brain magnetic resonance imaging (MRI) demonstrated a tumour in the left lateral ventricle (Fig. 1A). The tumour was subtotally removed and confirmed as a CN (Fig. 1B). For the residual tumour in the left lateral ventricle, gamma knife stereotactic radiosurgery (GK SRS) was done at fifteen months after the initial surgery (Fig. 1C). She had lived well without any overt symptom until tumour recurrence was found in the 4th ventricle in the follow-up MRI taken at 5 yr after initial surgery and 45 months after GK SRS (Fig. 1D). The tumour in the 4th ventricle was resected, which was proven the same pathology as the previous one (Fig. 1E, F).
The Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) of frozen central neurocytoma tumour cells from the initial and second operation demonstrated nestin products (Fig. 2). The sequences of upstream and downstream for nestin (209 bp) were 5'-CAGCTGGCGCACCTCAAGAT-3'(forward) and 5'-GGGAAGTTGGGCTC-AGGAC-3'(backward).
In vitro primary culture was done for both tumours in the left lateral ventricle and in the 4th ventricle in different culture media (Figs. 3-5). The tumour cells of CN in the lateral ventricle were cultured at first in the Dulbeco's Modified Essential Media (DMEM) (WellGENE, Daegu, Korea) with 20% fetal bovine serum (FBS) (Invitrogen, Gaithesburg, MD, USA). Within several days of primary culture in DMEM with 20% FBS, cultured cells became slightly enlarged and elongated (Fig. 3A). Several weeks after plating, cultured cells had increased in size with bipolar or multipolar cytoplasmic sprouting (Fig. 3B). Several months after plating, the cytoplasmic process of cultured cells was markedly enlarged with variable shapes (Fig. 3C). In contrast, the tumour cells of CN in the 4th ventricle were cultured at first when cultured in insulin, transferring, selenium and fibronectin (ITSFn) media with bFGF. Within several days of primary cell culture in ITSFn with bFGF, uniform small round cultured cells were dominant with their cytoplasmic processes (Fig. 3D). Several weeks after plating, the round cells had increased in size without elongation of their cytoplasm (Fig. 3E). Several months after plating, cultured cells displayed round cell morphology without prominent cytoplasmic process (Fig. 3F).
We had frozen the tumour cells obtained from the lateral ventricle at the initial surgery and cultured in DMEM with 20% FBS. Several months later the frozen tumour cells were thawed and sub-cultured again in different culture media: DMEM with 10% FBS (Fig. 4A-C) and ITSFn with bFGF (Fig. 4D-F). Within several days of culture in DMEM with 10% FBS, thawed cells returned to their original morphology at the freezing (Fig. 4A). At 6 and 20 days after thawing, the morphology of the tumour cells were the same as those in the original cells from the primary culture in DMEM with 20% FBS as shown in Fig. 3B (Fig. 4B, C). When cultured in ITSFn with bFGF, the thawed tumour cells returned to their original morphology at the freezing within several days (Fig. 4D). At 6 and 20 days after thawing, however, the morphology of the tumour cells were similar to those of primary culture tumour cells cultured in ITSFn with bFGF as shown in Fig. 3E (Fig. 4E, F).
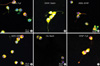
The immunocytochemical staining was performed for the tumour cells of CN in the fourth ventricle cultured in the ITSFn with bFGF (Fig. 5). Double labeling immunocytochemical studies demonstrated coexistence of neuronal, glial, and stem cell markers in the tumour cells of CN cultured in the ITSFn with bFGF. Nestin-positive cells were co-labeled with Tuj1 (Fig. 5A), Nestin-positive cells were co-labeled with Glial fibrillary acidic protein (GFAP) (Fig. 5B). A2B5-positive cells were co-labeled with GFAP (Fig. 5C), A2B5-positive cells were co-labeled with MAP2 (microtubule associated proteins) (Fig. 5D). O4-positive cells were co-labeled with neuronal nuclear protein (NeuN) (Fig. 5E), GFAP-positive cells were co-labeled with Tuj1 (Fig. 5F).
CN is a well-differentiated neuronal neoplasm, and was first recognized by Hassoun et al. in 1982 based on its immunohistochemical and ultrastructural features (5). Until now, however, the tumour origin and its characteristics were not completely understood. Several reports have demonstrated that CN may have the ability to favor differentiation into neurons and glia depending in the in vitro culture environment (7-10). In this study, we have found the stem cell nature of CN, in RT-PCR of frozen samples as well as in vitro primary culture.
RT-PCR from the frozen samples of both tumours obtained from the 4th ventricle and the lateral ventricle demonstrated nestin expression, although EM study of both tumours showed characteristics of neuronal origin tumours. Nestin is a class VI intermediate filament protein expressed in the proliferating progenitor cells in the developing CNS. The immunohistochemical staining revealed nestin expression in human brain of 6- to 40-week gestation (11). In this study, immunocytochemical staining of tumour cells of CN cultured in ITSFn media containing bFGF confirmed the co-existence of GFAP+/Nestin+, Nestin+/Tuj1+ cells. GFAP is a member of the intermediate filament protein family expressed primarily in glial cells such as astrocytes (12, 13). Radial glia cells, one type of neuronal stem cells in the developing brain also express the GFAP (14). Tuj1, a monoclonal antibody against neuronal class III β-tubulin is an early neuronal marker. MAP2 labels neuronal cells, their perikarya and neuronal dendrites. NeuN reacts with most neuronal cell types. Cell surface antibodies such as A2B5 and O4 specifically label cultured oligodendroglial progenitors or immature oligodendrocytes (15). Thus the co-existence of A2B5+/MAP2+, A2B5+/GFAP+ cells, GFAP+/Tuj1+ cells, and O4+/NeuN+ cells of cultured tumour cells of CN infers that the CN had the capacity to differentiate into both neurons and glia depending on the in vitro culture environment.
In contrast, immunocytochemical staining of tumour cells cultured for more than one month in DMEM with 20% serum mostly showed GFAP+ cells (data not shown). Thus these findings suggest that the tumour cells of CN could be driven into glial cell-lineage differentiation in in vitro primary culture depending on the environment cue.
Valdueza et al. (10) described that cultured cells of CN were positive for synaptophysin and GFAP after 24 hr of primary cell culture. Synaptophysin immunoreactivity, however, vanished over the next two in vitro passages, whereas GFAP expression persisted. Patt et al. (8) and Ishiuchi et al. (7) described that cultured cells of CN had characteristics intermediate between those of neurons and glia. Tsuchida et al. (9) reported that spatial cell growth and the presence of collagen, i.e., an extracellular matrix, may be necessary to retain neuronal differentiation in CN.
In the present study, the stemness of neuro-glial progenitor cells of CN was maintained in cells cultured in ITSFn media containing bFGF. bFGF is well known to play an important role in controlling the survival and proliferation of neural precursors (16, 17). Gensburger et al. (16) found that bFGF promoted the proliferation of neuronal precursor cells in primary cultures of embryonic rat. Kilpatrick et al. (17) observed that bFGF causes the proliferation of multipotent neuronal progenitors in fetal mice. Kato et al. (18) suggested that neurosphere formation by mouse embryonic stem cells is stimulated by cooperation between endogenous FGFs and cystatin C, by emphasizing the importance of endogenous bFGF produced from embryonic stem cells for neural lineage differentiation. Kim et al. (19) also reported that CN cells proliferated with the expression of the nestin gene in response to bFGF. In addition, Sim et al. (20) had mentioned interestingly that CN was especially notable for its differential overexpression of bFGF when compared with normal adult human ventricular zone cells.
These findings suggest that CN cells mimic uncommitted stem cells capable of differentiating into neuronal or glial cells according to culture conditions originating from the remnants of the subependymal germinal plate of the lateral ventricles.
Figures and Tables
Fig. 1
Imaging and pathological findings. (A) A T2 weighted brain MRI showed an inhomogeneous mass lesion in the left lateral ventricle. (B) The tumour cells were demonstrated as central neurocytoma (×200). (C) A T1 weighted brain MRI with contrast taken at 15 months after the initial surgery showed a residual mass lesion. (D) A T1 weighted brain MRI with contrast, taken at 45 months after gamma knife radiosurgery showed a ill-defined enhancing mass lesion in the left lateral ventricle (open arrow) and in the 4th ventricle (closed arrow). (E) The tumour in the 4th ventricle was confirmed as the same pathology as the previous one (×200). (F) An electron microscope demonstrated that the perikaryal cytoplasm and cytoplasmic processes have electron dense core neurosecretory granules, 10 nm-neural tubules (white arrow) and a synaptic junction (black arrow), the same finding as in the previous one (×8,000).
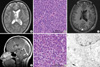
Fig. 2
RT-PCR of frozen central neurocytoma tumour cells from the initial and second operation demonstrated nestin products.
1, CN from the initial surgery; 2, CN from the second surgery; 3, 1 Kb DNA ladder.
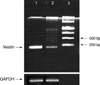
Fig. 3
Tumour cells of CN in the left lateral ventricle cultured in 20% DMEM (A-C) and tumour cells of CN in the 4th ventricle cultured in ITSFn with bFGF (D-F). (A) Within several days of primary culture in DMEM with 20% FBS, cultured cells became slightly enlarged and elongated (×200). (B) Several weeks after plating, cultured cells had increased in size with bipolar or multipolar cytoplasmic sprouting (×200). (C) Several months after plating, the cytoplasmic process of cultured cells was markedly enlarged with variable shapes (×400). (D) When cultured in ITSFn with bFGF, uniform small round cultured cells were dominant with their cytoplasmic processes within several days of in vitro primary culture of CN in the 4th ventricle (×200). (E) Several weeks after plating, the round cells had increased in size without elongation of their cytoplasm (×200). (F) Several months after plating, cultured cells displayed round cell morphology without prominent cytoplasmic process (×400).

Fig. 4
Morphological changes of tumour cells of CN in the left lateral ventricle cultured in different culture media after thawing; DMEM with 10% FBS (A-C) vs. ITSFn with bFGF (D-F) (×400). (A) Within several days of culture in DMEM with 10% FBS, thawed cells returned to their original morphology at freezing (×400). (B, C) The morphology of the tumour cells at 6 and 20 days after thawing were the same as those in the original cells from the primary culture in DMEM with 20% FBS as shown in Fig. 3B (×400). (D) When cultured in ITSFn with bFGF, the thawed tumour cells returned to their original morphology at freezing within several days (×400). (E, F) The morphology of the tumour cells at 6 and 20 days after thawing were similar to those of primary culture tumour cells cultured in ITSFn with bFGF as shown in Fig. 3E (×400).
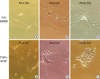
Fig. 5
Immunohistochemical staining of small round or bipolar tumour cells of CN in the 4th ventricle, cultured in ITSFn with bFGF. Double labeling immunocytochemical studies demonstrated strong positivity for both neuronal and glial markers. Nestin-positive cells were colabeled with Tuj1 (A, ×400), Nestin-positive cells were co-labeled with GFAP (B, ×400). A2B5-positive cells were co-labeled with GFAP (C, ×400), A2B5-positive cells were co-labeled with MAP2 (D, ×400). O4-positive cells were co-labeled with NeuN (E, ×400), GFAP-positive cells were co-labeled with Tuj1 (F, ×400).
References
1. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001. 414:105–111.

3. Doetsch F, Garcia_Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997. 17:5046–5061.

5. Hassoun J, Gambarelli D, Grisoli F, Pellet W, Salamon G, Pellissier JF, Toga M. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol. 1982. 56:151–156.
6. Von Deimling A, Kleihues P, Saremaslani P, Yasargil MG, Spoerri O, Sudhof TC, Wiestler OD. Histogenesis and differentiation potential of central neurocytomas. Lab Invest. 1991. 64:585–591.
7. Ishiuchi S, Nakazato Y, Iino M, Ozawa S, Tamura M, Ohye C. In vitro neuronal and glial production and differentiation of human central neurocytoma cells. J Neurosci Res. 1998. 51:526–535.

8. Patt S, Schmidt H, Labrakakis C, Weydt P, Fritsch M, Cervos-Navarro J, Kettenmann H. Human central neurocytoma cells show neuronal physiological properties in vitro. Acta Neuropathol. 1996. 91:209–214.

9. Tsuchida T, Yamada A, Yoshimura K, Kawamoto K. Ultrastructural characterization of central neurocytomas using collagen gel culture. Ultrastruct Pathol. 1998. 22:233–238.

10. Valdueza JM, Westphal M, Vortmeyer A, Muller D, Padberg B, Hermann HD. Central neurocytoma: clinical, immunohistologic, and biologic findings of a human neuroglial progenitor tumor. Surg Neurol. 1996. 45:49–56.

11. Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD, Trojanowski JQ. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992. 66:303–313.
12. Raff MC, Miller RH, Noble MA. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983. 303:390–396.

13. Tardy M, Fages C, Riol H, LePrince G, Rataboul P, Charriere-Bertrand C, Nunez J. Developmental expression of the glial fibrillary acidic protein mRNA in the central nervous system and in cultured astrocytes. J Neurochem. 1989. 52:162–167.

14. Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting revealed a neuronal lineage. Development. 2000. 127:5253–5263.
15. Satoh J, Kim SU. Ganglioside markers GD3, GD2, and A2B5 in fetal human neurons and glial cells in culture. Dev Neurosci. 1995. 17:137–148.

16. Gensburger C, Capo M, Deloulme JC, Sensenbrenner M. Influence of basic fibroblast growth factor on the development of cholinoreceptive neurons from fetal rat cerebrum in culture. Dev Neurosci. 1992. 14:278–281.
17. Kilpatrick TJ, Bartlett PF. Cloning and growth of multipotential neural precursors: requirements for proliferation and differentiation. Neuron. 1993. 10:255–265.

18. Kato T, Heike T, Okawa K, Haruyama M, Shiraishi K, Yoshimoto M, Nagato M, Shibata M, Kumada T, Yamanaka Y, Hattori H, Nakahata T. A neurosphere-derived factor, cystatin C, supports differentiation of ES cells into neural stem cells. Proc Natl Acad Sci USA. 2006. 103:6019–6024.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download