Abstract
Neural stem cells (NSCs) have mainly been applied to neurodegeneration in some medically intractable neurologic diseases. In this study, we established a novel NSC line and investigated the cytotoxic responses of NSCs to exogenous neurotoxicants, glutamates and reactive oxygen species (ROS). A multipotent NSC line, B2A1 cells, was established from long-term primary cultures of oligodendrocyte-enriched cells from an adult BALB/c mouse brain. B2A1 cells could be differentiated into neuronal, astrocytic and oligodendroglial lineages. The cells also expressed genotypic mRNA messages for both neural progenitor cells and differentiated neuronoglial cells. B2A1 cells treated with hydrogen peroxide and L-buthionine-(S,R)-sulfoximine underwent 30-40% cell death, while B2A1 cells treated with glutamate and kainate showed 25-35% cell death. Cytopathologic changes consisting of swollen cell bodies, loss of cytoplasmic processes, and nuclear chromatin disintegration, developed after exposure to both ROS and excitotoxic chemicals. These results suggest that B2A1 cells may be useful in the study of NSC biology and may constitute an effective neurotoxicity screening system for ROS and excitotoxic chemicals.
The capacity of stem cells to express multipotentiality in differentiation and self-renewal properties has been well established in cells from many organs, but particularly the hematopoietic system (1). This is also true of cells from the central nervous system (2). In recent years, there has been an increased interest in the development of adult tissue-derived multipotent stem cells for the treatment of human diseases, rather than those of the fertilized ovum or early totipotent or blastocyst-derived embryonic stem cells (3). The potential use of totipotent or embryonic stem cells presents a variety of ethical problems.
The presence of neural stem cells (NSCs) has already been reported in the mammalian embryonic cerebral cortex. These cells are capable of producing three principal cell types of the central nervous system (CNS); neurons, astrocytes and oligodendrocytes (4). These precursor cells, generally located in the subventricular zone (SVZ), have also been identified in adult rat and human brains (4, 5). Multiple physiologic factors, such as growth factors, cytokines and adhesion molecules, have been shown to be involved in intrinsic and extrinsic control of NSCs' self-renewal and differentiation (6). Research applications utilizing NSCs are focused mainly on identification of inductive factors and mediators in human neurogenesis, the determination of gene function in neuronal and glial differentiation, the modeling of neurodegeneration, pharmaceutical discovery, and gene or cell replacement therapy (2, 7, 8). Recently, in vitro neurotoxicity studies using NSCs instead of neuronal cell cultures have been reported (9, 10).
In a previous study, a glial precursor cell line, designated as B2 cells, was generated from primary cultures of oligodendrocytes/astrocytes isolated from an adult mouse brain (11).
Using our established B2 cells, we performed the present study to establish a clonal NSC line and investigate whether the cells were useful for in vitro neurotoxicity screening tests previously limited to neuronal cell cultures.
B2 cells selected from undifferentiated cell clusters present in oligodendrocyte-enriched cultures were used as parent cells, and were prepared from whole brains of 12-week-old BALB/c mice. The detailed methods for selecting B2 cells and their characterization have been described previously (11). Briefly, during the first week of culture, about 90% of the cells exhibit surface immunolabeling of galactocerebroside (GalC), a cell type-specific marker for oligodendrocytes. After 4 weeks in vitro, the proportion of oligodendrocytes in oligodendroyte-enriched cultures is reduced to 40-60%. Non-oligodendroglial cells, including 10-20% glial fibrillary acidic protein (GFAP)-positive astrocytes, and 30-40% small bipolar cells morphologically distinct from oligodendrocytes or astrocytes, populate the cultures. These small bipolar cells aggregate each other, and form a cell cluster or sphere, and have 8 µm cell bodies and 20-30 µm cell processes. The cells are harvested by a brief treatment for 10 min with phosphate buffered saline (PBS) containing 0.1% trypsin and 1 mM ethylenediaminetetraacetic acid (EDTA). They are then suspended in culture medium, and replated into T75 culture flasks. After 3 months with 6 serial passages, the culture consisted of a morphologically homogeneous cell population, designated as B2 cells. They were further maintained for 6 months with 25 serial passages in a culture medium, containing 10% horse serum (HS). The identity of mouse-derived cells was verified by karyotype analysis with G-banding technique (12). The total chromosome number of B2 cells was 40, which is identical to normal somatic cells of BALB/c mice.
B2 cell cultures were dissociated into single cells, suspended in 10% HS-containing medium, and plated in the absence of feeder cells in a 96-well microtiter plate at a density of one cell/well. The medium was renewed by half once per week. After three weeks, well-separated colonies were picked for further expansion. This procedure was repeated once more. Only one out of the 28 clones, designated as B2A1, successfully generated and expanded. Stock cultures of B2A1 cells were grown in 75 cm2 tissue culture flasks in the serum-free chemically defined medium DM4 supplemented with 10% fetal bovine serum (FBS) (13). The DM4 medium consisted of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 µg/mL insulin, 10 µg/mL of transferrin, 0.3 nM of triiodothyronine, 30 nM of sodium selenite and 50 nM of hydrocortisone. All chemicals described above, except for insulin (Novo Laboratories, Willowdale, ON, Canada), were obtained from Sigma (St. Louis, MO, USA). Cultures were maintained at 37℃ in a humidified incubator containing 5% CO2, and the cells were passed once a week.
For immunocytochemical studies, B2A1 cells were plated on poly-L-lysine (PL)-coated 9 mm round Aclar coverslips at a density of 1×104 cells/coverslip. The cells were also cultured in the medium for five days, fixed in cold acid alcohol (5% acetic acid in 95% ethanol) for 15 min at -20℃, and incubated with normal goat serum for 20 min at room temperature (then processed for immunocytochemistry). To identify neural cell phenotypes, cell cultures were processed for immunostaining using cell type-specific markers: nestin for neural stem cells; low molecular weight neurofilament protein (NF-L) and β tubulin III for neurons; GFAP for astrocytes; GalC and myelin basic protein (MBP) for oligodendrocytes; and, Ricinus communis agglutinin-1 lectin (RCA-1) for microglia. The cultures were incubated with primary antibodies, followed by biotinylated secondary antibodies and avidin-biotin complex (ABC, Vector, Burlingame, CA, USA) and visualized with 3-amino-9-ethyl carbazole chromogen (AEC, Sigma). The antibodies utilized for immunochemical characterization of neural cell types are listed in Table 1.
To investigate effects upon neural cell differentiation by various growth factors and pharmacological agents in B2A1 cells, the cells were plated on PL-coated coverslips at a density of 1×104 cells/coverslip. They were incubated for 72 hr in DMEM (DM4), with triiodothyronine and hydrocortisone supplement, in the presence of one of the following: 10% FBS, with various mixtures of 10 µg/mL of insulin, 10 µg/mL of transferrin, 30 nM selenite (ITS) (Sigma), 1mM dibutyryl cyclic AMP (dbcAMP) (Sigma), 10 µM retinoic acid (RA) (Sigma), 100 ng/mL of recombinant human basic fibroblast growth factor (bFGF) (GIBCO BRL, Gaithersburg, MD, USA), 100 ng/mL of recombinant human platelet-derived growth factor (PDGF) AA (Upstate Biotechnology, Lake Placid, NY, USA), 10 ng/mL of leukocyte inhibitory factor (LIF, Sigma) or 100 nM phorbol 12-myristate 13-acetate (PMA, Sigma). The immunocytochemical stains for nestin, β tubulin III, GFAP and GalC were processed from the cultures as described above.
For reverase transcriptase-polymerase chain reaction (RT-PCR) analysis, B2A1 cells were plated on 6 well plates at a density of 5×105 cells/well and incubated for 5 days in DM4 with addition of one of the above cytokines. RT-PCR was performed in the cells harvested from each well using the oligonucleotide primers listed in Table 2 (14, 15). The expression of nestin, Notch1, and platelet-derived growth factor receptor-α (PDGFR-α) for neural stem cell markers were determined. To confirm the neuronal property of B2A1 cells, complementary DNA (cDNA) was amplified 35 PCR cycles for NF-L. In addition, we looked for other CNS cell type markers, GFAP for astrocytes, and MBP for oligodendrocytes. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was used as a reaction standard. Total RNA was extracted from each sample using TRIzol reagent (GIBCO-BRL). cDNA templates from each sample were prepared from 2 µg of total RNA primed with oligo dT primers (Pharmacia, Gaithersberg, MD, USA) using 400 units of Moloney murine leukemia virus (MMLV) reverse transcriptase (GIBCO-BRL) followed by 35-40 PCR amplification cycles (94℃ for 30 sec, annealing at 55℃ for 60 sec and extension at 72℃ for 90 sec). Ten microliters of each PCR product were analyzed by 1.5% agarose gel electrophoresis. Authenticity of bands was determined by selective enzyme digestion.
For toxicity studies, B2A1 cells were seeded on both Aclar coverslips and 96-well plates at a density of 1×104 cells per coverslip or well in DMEM containing 10% fetal bovine serum, 5 mg/mL glucose, 25 µg/mL of gentamicin, and 2.5 µg/mL of amphotericin B, and cultured for 7 days. The cultures were changed to a medium containing no fetal bovine serum 24 hr prior to cytotoxic treatment, and cells were exposed to the chemicals for an additional 24 hr. All chemicals were purchased from Sigma and the concentrations used for treatment were: glutamate (20 µM-2 mM), kainate (20 µM-2 mM), hydrogen peroxide (H2O2, 100 µM-1 mM), and buthionine sulfoximine (BSO) (1-100 µM).
Cytotoxicity was evaluated by cytopathologic changes and cell viability of B2A1 cells after treatment with exogenous ROS and excitotoxic chemicals. Cytopathologic features of B2A1 cells on Aclar coverslips were examined using a phase-contrast inverted microscope.
In order to measure viability of the B2A1 cells, the 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay was performed (16). This method is based on the cellular reduction of tetrazolium salt MTT (Sigma) by the mitochondrial dehydrogenase of viable cells, which results in formation of a blue formazan product which can then be measured with a spectrophotometer. In brief, MTT was dissolved in DMEM at a concentration of 5 mg/mL, filtered through a membrane filter (45 µm in diameter), and stored at 4℃ in an aluminum foil-sealed tube. Following addition of MTT stock solution into the cultures in 96 well plates (10 µL per 100 µL medium), the cultures were incubated for 4 hr at 37℃. A portion of the MTT was converted to an insoluble bluish purple formazan by cleavage of the tetrazolium ring by cellular dehydrogenase enzymes. The medium was removed, and the formazan product was dissolved with 100 µL of acid-isopropanol (0.04N hydrochloride in isopropanol) per well. Optical density was measured using an ELISA plate-reader at a wave length of 570 nm. The test was repeated five times. Statistical analysis was done using a one way ANOVA, followed by Dunnett's multiple comparison test. The level of significance was set at P<0.05. All statistical analyses were conducted by the SPSS 15.0 statistical software program (SPSS 15.0, Chicago, IL, USA).
In serum-free DM4 medium, B2A1 cells were expressed as either bipolar or tripolar cells with a 7-8 µm cell body size. They had a tendency to grow individually or to aggregate into small clumps (Fig. 1A). Nestin immunopositivity was 97.5% in the cells (Fig. 1B).
A limited number of cells expressed cell type specific markers for neurons, such as NF-L (2.1%) and β tubulin III (1.9%) (Fig. 1C). In addition, other CNS cell markers, including GFAP (astrocyte marker), and GalC and MBP (oligodendrocyte markers) were positive using immunocytochemistry in 2.9%, 2.3%, and 1.7% of the cells, respectively (Fig. 1D). RCA-1 as a microglial marker had no immunoreactivity in the cells. The above immunoreactivities for CNS cell type specific markers are summarized in Table 3.
Cells reacting positively for nestin, β tubulin III, GFAP and GalC per total B2A1 cells following incubation in DM4 serum-free medium, treated with serum or various growth factors, are presented in Table 3. When incubated in 10% FBS, ITS, dbcAMP, bFGF, PDGF, LIF or PMA-containing culture medium, immunoreactivity for nestin was positive in 85.4-95.8% of B2A1 cells (Fig. 2A). The proportion of nestin positive cells decreased in RA-containing culture medium (84.7%), while β tubulin III positive cells were significantly increased (15.6%) (P<0.05). When treated with PDGF, 10% FBS, dbcAMP, RA or bFGF, β tubulin III was expressed in fewer cells (2.1-2.8%). Only a few cells were positive for β tubulin III (less than 0.5%) in ITS, LIF, or PMA treated culture medium (Fig. 2B).
When incubated in dbcAMP or PDGF-containing culture medium, GFAP immunolabeling was seen in 14.3-16.0% of B2A1 cells, while 10% FBS, ITS, RA, bFGF or LIF-containing medium was expressed in just 2.2-7.1% (P<0.01). Immunopositivity for GFAP was seen in 2.2% of B2A1 cells cultured in PMA-containing medium (Fig. 2C). When cultured in 10% cAMP or PDGF-containing medium, GalC positive cells were seen in 7.3-8.9% of B2A1 cells, while only 2.9-5.8% of the cells were positive in 10% FBS, RA, ITS, bFGF, or PMA-containing medium (P<0.05) (Fig. 2D).
To determine whether B2A1 cells can express markers of neural stem cells, as well as of early differentiating neurons, astrocytes and oligodendrocytes, RT-PCR was performed in cultured cells treated with various cytokines. Expression levels were compared with the control expression of the housekeeping gene G3PDH, and the data are summarized in Fig. 3. Stem cell or early precursor cell markers of CNS origin (nestin, Notch1, PDGFR-α), neuronal markers for NF-L, astrocytic markers for GFAP, and oligodendroglial markers for MBP, were consistently expressed. Even though the intensity of expression demonstrated some variation using treated serum or growth factors, the expression of genotypic markers correlated relatively well with immunocytochemical phenotypes.
Compared with control cells (Fig. 4A), swelling of cell bodies was the initial common cytopathologic hallmark in B2A1 cells treated with the cytotoxic chemicals for 4-6 hr. This was followed by fragmentation and loss of neurites, loss of cell to cell contact. Nuclear homogenization was then seen during the longer incubation time of 24 hr. Cells treated with H2O2 (500 µM) or BSO (1-100 µM) developed swelling of cell bodies with focally expanding parts of cytoplasmic process (axon hillocks), vacuolar changes with increased granularity of cytoplasm and cytoplasmic processes (neuritis) (Fig. 4B), and progressive disintegration of the nuclear chromatin (Fig. 4C). Glutamate or kainate-treated cells (20-2,000 µM) also showed swelling of cell bodies at 4-6 hr; however, swelling was limited to the cytoplasm of the cells and was more homogenous when compared with H2O2 or BSO-treated cells. As time increased, the nuclear chromatin and cytoplasm of the cells also disintegrated, and dead cells formed homogenous round bodies (Fig. 4D).
In order to investigate the relationship of H2O2 exposure to B2A1 cell death, the concentration of H2O2 which induces cell death was measured using the MTT test (Fig. 5). There was no significant cell death seen 24 hr after treatment with 1-100 µM H2O2 (Fig. 5A). However, viable cells were significantly reduced 24 hr after the addition of 500 µM H2O2 (67.7±2.6%) and 1 µM BSO (65.6±2.9%) (P<0.01 compared to control cultures). Viable cells were also reduced to 61.3-65.6% 24 hr after treatment with 1-100 µM BSO (Fig. 5B). In 20-2,000 µM glutamate or kainate group treated for 24 hr, viable cells were decreased to 66.6-74.4% (Fig. 5C, D) (P<0.01 compared to control cultures). A dose-response pattern of cell death was not present in the BSO, glutamate or kainate treated groups in this experiment.
In this study, an NSC line designated as B2A1 was generated from isolated adult mouse brain cells by use of a long term, two-step culture process. The first step generated B2 cells from primary cultures of oligodendrocyte-enriched fractions. The B2 cells were immunopositive for vimentin, the major intermediate filament protein of immature neuroectodermal cells (17). The B2 cells did not express any cell type-specific markers for neuronal, microglial, or endothelial cells. When cultured in the presence of dbcAMP, cells were immunopositive for vimentin, GFAP, O4, and GalC. These immunocytochemical features suggest that B2 cells were composed of glial precursor cells mixed with immature neuroectodermal cells.
The second step in development generated B2A1 cells from focal aggregates of B2 cells using limited dilution. The B2A1 cells demonstrated several characteristics of neural stem cells in the following aspects.
First, most of the cells (95.7%) were immunopositive for nestin, an intermediate filament protein specifically expressed in NSCs from murine CNS (18). Second, B2A1 cells maintained NSC markers, and could differentiate into neurons, astrocytes and oligodendrocytes when cultured in the presence of growth factors, such as ITS, dbcAMP, RA, bFGF, PDGF, LIF, and PMA. Neuronal differentiation was markedly increased in the presence of RA. Astrocytic differentiation significantly increased with the addition of dbcAMP and PDGF, while oligodendrocytes were increased by PDGF treatment. These immunocytochemical features suggest that B2A1 cells have the multipotentiality for differentiating into cells of the CNS. Third, genotypic expression of B2A1 cells as seen with RT-PCR analysis demonstrates mRNA for nestin, Notch1, and PDGFR-α, all of which are genomic markers for undifferentiated neuroepithelial cells (18-20). Forth, other differentiated neural cell mRNAs, such as NF-L, GFAP, and MBP, were identified in B2A1 cells. There was a good correlation between mRNA expressions seen with RT-PCR and the immunocytochemical features noted in this study.
Recent molecular genetic studies show that a number of molecules are expressed in the early stages of embryonic neural development. These include transcription factors, such as Brain1 (Brn1), Numb, NeuroD, and downstream effectors of Notch1, Hes1, and Presenilin (18-22). Notch1 is expressed in progenitor cells within the subventricular zone in mice, suggesting that there is a biologic role in both progenitor cell proliferation and neuronal differentiation during mammalian cortical neurogenesis (21). PDGFR-α expression has primarily been described in oligodendrocyte precursors during early CNS development (20). In a recent study, PDGFR-α was identified in neuroepithelial cells of the neural plate at day 8.5 in embryonic mice, as well as in developing granule cells in the postnatal cerebellum, in Purkinje cells in the adult cerebellum and on the processes of developing dorsal root ganglion cells (20). These observations suggest that PDGFR-α is another marker of undifferentiated cells, which are subsequently able to differentiate into neurons and glial cells. When B2A1 cells were cultured in PDGF in this study, increased fraction of differentiated neurons, astrocytes, and oligodendrocytes were identified. The cytologic differentiation of B2A1 cells might thus be due to PDGFR-α.
In order to study stem cells at any level, one must be able to purify, or at least enrich for, stem cells from the target tissue or organ. One method of enriching for particular cell types is by use of fluorescence-activated cell sorting (FACS) or magnetic-activated cell sorting (MACS) (23). These techniques rely on the ability of antibodies to bind to proteins expressed on the cell surface, and they have commonly been used to isolate hematopoietic stem cells (HSC). Another method used to isolate stem cells is with the use of long-term cell culture, which was applied in this study to generate an NSC line (24). Briefly, whole cell populations are isolated from the tissue of interest and then grown in cultures which are conducive to the proliferation of stem cells and the maintenance of stem cell characteristics. This method has been used both for the purification of NSCs and of mesenchymal stem cells (MSCs).
There have been a few studies concerning the generation of multipotent neural stem cells in mice. A CNS-derived, conditionally immortalized, neural progenitor cell line (CINP) was generated to make nerve growth factor (NGF)-secreting cells using a retroviral vector (25). Other NSCs have been purified from adult mouse brain by flow cytometry, and used to study their functional ability to generate neural and nonneural cells (26). Another multipotent neural progenitor cell (2Y6f1) has been cloned from the cerebellum of an adult p53-/- mouse (27). The B2A1 cells used in our studies reported here were established from long term primary cultures derived from BALB/c adult mice brains maintained within normal physiologic conditions. This has supported development of a new cell line useful for the study of neural stem cell biology.
In the biochemical process that leads to neuronal cell death, ROS (superoxide, hydroxyl radical and hydrogen peroxide) and the excitatory neurotransmitter glutamate have been shown to have a relation to major neurological diseases, such as stroke, epilepsy, trauma, and neurodegeneration (28). This present study revealed levels of 25-30% cell death in B2A1 cultures that were treated with 20-2,000 µM glutamate or kainite for 24 hr, compared to control groups. High concentrations of glutamate (10 mM or more) has been known to be a major contributing mechanism for causing neuronal damage in the CNS, due to disruption of normal intracellular oxygen radical homeostasis. Under conditions such as cerebral ischemia, extracellular glutamate levels increase 8 fold, which results in a marked decrease in brain GSH levels due to the blocking of cystine uptake (29).
In addition to excitotoxic cell death in B2A1 cells, cell death from H2O2 and BSO was established in this study. B2A1 cell death occurred in a dose-dependent manner in cultures treated with more than 100 µM H2O2, whereas rates of 30-35% cell death occurred in 1-100 µM BSO. It is generally accepted that BSO is an irreversible inhibitor of glutathione synthase. The cytotoxic effect of BSO in B2A1 cells treated for 24 hr might be related to decreases in GSH synthesis.
There were no significant differences seen between excitotoxic and ROS injury in the cytopathologic changes of B2A1 cells. Cells treated with glutamate or kainate, develop a swollen cell body with loss of neurites, and then nuclear disintegration is noted. However, the cells treated with H2O2 or BSO display swelling of the cell body and neurites, with vacuole formation. These cytopathologic features suggest that excitotoxic chemicals mainly act upon cell surface receptors, resulting in Ca2+ overload, while ROS mainly act by direct damage to the cell membrane. Nevertheless, this speculation needs to be clarified through further studies based on ultrastructural or biochemical evidences.
In summary, our present study demonstrates the establishment of an NSC line which differentiates into neurons, astrocytes, and oligodendrocytes following treatment with growth factors. Cytotoxic cell death due to ROS and excitotoxic chemicals occurred. These data strongly suggest that B2A1 cells might be useful both for the neurobiologic study of NSC and as a neurotoxicity screening system utilizing primary neuronal cultures of the CNS.
Figures and Tables
Fig. 1
Cytologic characteristics of B2A1 cells include bi- or tripolar cells (A, phase contrast microscopy), which are immunopositive for nestin (B), β tubulin III (C), and GFAP (D) (×200).
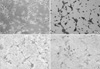
Fig. 2
Multipotential differentiation of B2A1 cells by treatment with growth factors. Most cells were immunopositive for nestin in any culture condition (A). Cells immunopositive for β tubulin III significantly increased in RA-containing media (B), while GFAP (C) and GalC (D) positive cells increased markedly in c-AMP or PDGF-containing media.

Fig. 3
Gene expression of cell type specific markers studied by RT-PCR in B2A1 cells. The cells expressed both neural progenitor cell markers (nestin, Notch1, PDGFR-α) and differentiated cell markers for neurons (NF-L), astrocytes (GFAP), and oligodendrocytes (MBP). The expression levels vary depending upon the level of growth factors added.

Fig. 4
Cytopathologic features of B2A1 cells. Compared with the control cells (A), cells treated with BSO (10 µM) show swelling of cell bodies with axon hillocks, vacuolar changes with increased granularity of the cytoplasm at 4 hr (B), loss of neurites, progressive disintegration of nuclear chromatin with homogenization, and loss of cell to cell contact at 24 hr (C). Glutamate or kainate-treated cells (20 µM) also show swelling of cell bodies and loss of neurites at 12 hr (D) (×200).
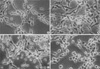
Fig. 5
Effect of H2O2 (A), BSO (B), glutamate (C) and kainate (D) treatment for 24 hr, measured by the MTT test. Data are shown as mean±standard deviation. The number of viable cells are significantly decreased after the addition of 500 µM H2O2 along the stepwise increase of doses. However, a dose-response pattern is not observed in the BSO, glutamate, or kainite treated groups.

References
1. Metcalf D. Concise review: hematopoietic stem cells and tissue stem cells: current concepts and unanswered questions. Stem Cells. 2007. 25:2390–2395.

2. Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU, Snyder EY. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998. 16:1033–1039.
3. Mimeault M, Batra SK. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008. 4:27–49.

4. Davis AA, Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994. 372:263–266.

5. Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O'Brien TF, Kusakabe M, Steindler DA. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999. 156:333–344.

6. Anderson DJ. Stem cells and pattern formation in the nervous system: the possible versus the actual. Neuron. 2001. 30:19–35.
7. Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002. 20:1103–1110.

8. Ryu JK, Kim J, Cho SJ, Hatori K, Nagai A, Choi HB, Lee MC, Mc-Larnon JG, Kim SU. Proactive transplantation of human neural stem cells prevents degeneration of striatal neurons in a rat model of Huntington disease. Neurobiol Dis. 2004. 16:68–77.

9. Davidovics Z, DiCicco-Bloom E. Moderate lead exposure elicits neurotrophic effects in cerebral cortical precursor cells in culture. J Neurosci Res. 2005. 80:817–825.

10. Breier JM, Radio NM, Mundy WR, Shafer TJ. Development of a high-throughput screening assay for chemical effects on proliferation and viability of immortalized human neural progenitor cells. Toxicol Sci. 2008. 105:119–133.

11. Satoh J, Tabira T, Kim SU. Rapidly proliferating glial cells isolated from adult mouse brain have a differentiative capacity in response to cyclic AMP. Neurosci Res. 1994. 20:175–184.

12. Rey JA, Bello MJ, De Campos JM, Kusak ME, Moreno S. Cytogenetic analysis in human neurinomas. Cancer Genet Cytogenet. 1987. 28:187–188.

13. Kim SU, Stern J, Kim MW, Pleasure DE. Culture of purified rat astrocytes in serum-free medium supplemented with mitogen. Brain Res. 1983. 274:79–86.

14. Cai J, Wu Y, Mirua T, Pierce JL, Lucero MT, Albertine KH, Spangrude GJ, Rao MS. Properties of a fetal multipotent neural stem cell (NEP cell). Dev Biol. 2002. 251:221–240.

15. Nagai A, Suzuki Y, Baek SY, Lee KS, Lee MC, McLarnon JG, Kim SU. Generation and characterization of human hybrid neurons produced between embryonic CNS neurons and neuroblastoma cells. Neurobiol Dis. 2002. 11:184–198.

16. Lee MC, Chung YT, Lee JH, Jung JJ, Kim HS, Kim SU. Antioxidant effect of melatonin in human retinal neuron cultures. Exp Neurol. 2001. 172:407–415.

17. Bignami A, Raju T, Dahl D. Localization of vimentin, the nonspecific intermediate filament protein, in embryonal glia and in early differentiating neurons. In vivo and in vitro immunofluorescence study of the rat embryo with vimentin and neurofilament antisera. Dev Biol. 1982. 91:286–295.
18. Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990. 60:585–595.

19. Zhong W, Jiang MM, Weinmaster G, Jan LY, Jan YN. Differential expression of mammalian Numb, Numblike and Notch1 suggests distinct roles during mouse cortical neurogenesis. Development. 1997. 124:1887–1897.

20. Andrae J, Hansson I, Afink GB, Nister M. Platelet-derived growth factor receptor-alpha in ventricular zone cells and in developing neurons. Mol Cell Neurosci. 2001. 17:1001–1013.
21. Kageyama R, Ohtsuka T. The Notch-Hes pathway in mammalian neural development. Cell Res. 1999. 9:179–188.

22. Yang X, Handler M, Shen J. Role of presenilin-1 in murine neural development. Ann N Y Acad Sci. 2000. 920:165–170.

23. Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996. 183:1797–1806.

24. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.

25. Martinez-Serrano A, Lundberg C, Horellou P, Fischer W, Bentlage C, Campbell K, McKay RD, Mallet J, Bjorklund A. CNS-derived neural progenitor cells for gene transfer of nerve growth factor to the adult rat brain: complete rescue of axotomized cholinergic neurons after transplantation into the septum. J Neurosci. 1995. 15:5668–5680.

26. Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001. 412:736–739.

27. Ohkawara B, Okuno M, Ishii T, Horiuchi M, Tomooka Y. Characterization of a multipotent neural progenitor cell line cloned from an adult p53-/- mouse cerebellum. Brain Res. 2003. 959:11–19.

28. Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993. 262:689–695.

29. Shivakumar BR, Kolluri SV, Ravindranath V. Glutathione and protein thiol homeostasis in brain during reperfusion after cerebral ischemia. J Pharmacol Exp Ther. 1995. 274:1167–1173.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download