Abstract
The bronchial pathology of asymptomatic airway hyperreponsiveness (AHR) subjects is not well understood, and the role of atopy in the development of airway remodeling is unclear. The aim of this study was to evaluate whether atopy is associated with airway remodeling in asymptomatic AHR subjects. Five groups, i.e., atopic or non-atopic subjects with asymptomatic AHR, atopic or non-atopic healthy controls, and subjects with mild atopic asthma, were evaluated by bronchoscopic biopsy. By electron microscopy, mean reticular basement membrane (RBM) thicknesses were 4.3±1.7 µm, 3.4±1.8 µm, 2.5±1.5 µm, 2.6±1.1 µm, and 2.3±1.2 µm in the mild atopic asthma, atopic and non-atopic asymptomatic AHR, atopic and non-atopic control groups, respectively (p=0.002). RBM thicknesses were significantly higher in the mild atopic asthma group and in the atopic asymptomatic AHR group than in the other three groups (p=0.048). No significant difference in RBM thickness was observed between the atopic asymptomatic AHR group and the mild atopic asthma group (p>0.05), nor between non-atopic asymptomatic AHR group and the two control groups (p>0.05). By light microscopy, subepithelial layer thicknesses between the groups showed the same results. These findings suggest that RBM thickening occurs in subjects with atopic asymptomatic AHR, and that atopy plays an important role in airway remodeling.
Airway hyperresponsiveness (AHR) is a characteristic feature of asthma. It reflects a tendency for bronchi to over-narrow in response to various provocative stimuli (1). Asthma is usually diagnosed in patients with both AHR and respiratory symptoms, such as, dyspnea, cough, wheeze, or chest tightness. Although AHR is a cardinal feature in asthma, a significant proportion of individuals without past or present history of asthma and other respiratory diseases show an increased physiologic airway response to agents such as histamine or methacholine or to stimuli such as exercise with no respiratory symptoms (1). This is usually referred to as asymptomatic AHR.
The prevalence of AHR in the general population varies from 4 to 35% (1, 2). In our previous study, the prevalence of AHR in adults was determined to be 6.6% (3). However, the prevalence of asymptomatic AHR in the general population has not been well established and varies greatly from one survey to another. The majority of surveys have reported a prevalence of under 15% in adults (1, 2). In children, reported prevalences vary in much the same way, i.e., from 8 to 33% (2).
Asymptomatic AHR is believed to be associated with airway inflammation and remodeling (4, 5). However, few studies have examined bronchial inflammation and remodeling in asymptomatic AHR subjects. Laprise et al. reported that findings of airway inflammation in an asymptomatic AHR group showed epithelial desquamation and focal irregular subepithelial fibrosis, which were similar to those of asthma by bronchial biopsy (4). In addition, median percentages of eosinophils in induced sputum were found to be similar in asthmatic and asymptomatic AHR (5).
Atopy has also been associated with airway remodeling and reticular basement membrane (RBM) thickening (6, 7), the latter of which was found to be significantly thicker in atopic asthma than in non-atopic asthma (8).
Asymptomatic AHR and atopy seem to be independent risk factors for the development of asthma (1, 4, 6, 8-11). In one study, atopic subjects with asymptomatic AHR showed a greater frequency of asthma symptom development than normoresponsive subjects over a 3-yr period (9). Moreover, asymptomatic children with AHR were found to be more likely to develop asthma and atopy later in life than asymptomatic children without AHR (10).
The purpose of this study was to evaluate whether RBM thickening begins in asymptomatic AHR subjects and whether atopy can influence airway remodeling in asymptomatic AHR subjects.
Twenty eight non-smoking volunteers were recruited. A questionnaire including asthma, allergy, and smoking history was administered. Methacholine bronchial provocation test and skin prick test were done. With given informed consent, a bronchoscopic examination was done. These 28 study subjects were then divided into five groups according to respiratory symptoms, and the presences of airway hyperresponsiveness and atopy, i.e., atopic or nonatopic subjects with asymptomatic AHR, mild atopic asthmatics, atopic or nonatopic controls. Subjects' ages ranged from 22 to 32 yr.
Twelve subjects with asymptomatic AHR had a provocative concentration of methacholine causing a 20% fall in forced expiratory volume in one second (PC20) of ≤16 mg/ mL. All denied any past or present symptoms suggestive of asthma (including intermittent dyspnea, wheezing, or a recurrent cough) or rhinitis. Seven of these were atopic and 5 were non-atopic. None of these 12 had contracted a respiratory infection during the month preceding this study.
A mild atopic asthma group of two subjects with current mild intermittent symptoms used only intermittent bronchodilator inhalation. Both were atopic without rhinitis and were stable at the time of the study, and neither had experienced a respiratory infection nor asthma exacerbation during the month preceding this study. Asthma was defined according to the criteria proposed by the American Thoracic Society(ATS) (12).
Two control groups were also studied; an atopic control group of 7 subjects, and a non-atopic control group of 7 subjects. Selection criteria for these control groups were PC20 >16 mg/mL, no current or past symptoms suggesting asthma, rhinitis or other respiratory disease, no medication and no respiratory infection during the prior month.
This study was approved by the hospital medical ethics committee of Seoul National University Hospital, and all participants gave informed consent.
All five study groups were evaluated at baseline. Characteristics of subjects and symptoms were documented according to responses to a detailed structured questionnaire.
Skin prick tests were performed using a battery of 16 common inhalant allergens, which included Dermatophagoides farinae and D. pteronyssinus, the two-spotted mite, Tyrophagus, cat, cockroach, ragweed, mugwort, mixed trees, mixed grasses, and mixed fungi (Allergopharma, Reinbek, Germany), as previously described (13). Normal saline and histamine dihydrochloride (Allergopharma) were used as negative and positive controls, respectively. Atopy was defined as the presence of at least one positive response to skin prick test (wheal diameter of allergen/wheal diameter of histamine ≥1 and mean wheal size ≥3 mm). Inhalant allergens were divided into five categories: house dust mite, tree pollens, grass pollens, animal danders, and molds. Atopic index was defined as the number of inhalant allergen categories (0-5) to which a patient showed at least one positive response on skin prick test (4).
Methacholine bronchial provocation tests were performed as previously described (14). AHR was defined as the provocative concentration of methacholine causing a 20% decline in FEV1 (PC20) of ≤16 mg/mL. PC20 was obtained by interpolating dose-response curves to a 20% fall in FEV1.
Study subjects underwent bronchoscopy at a day care unit using a standard set up, as described by the American Thoracic Society (15). They were admitted in the morning after an overnight fast. After making baseline observations, all subjects received 2.5 mg of nebulized salbutamol and 600µg of atropine intramuscularly as premedication. A lidocaine spray was applied to the oropharynx, and 4% lidocaine was installed via a bronchoscope to the vocal cords. Additional 2% lidocaine was administered to the tracheobronchial tree. Oxygen at 2 L/min was delivered during the procedure and subjects were monitored by pulse oximetry. Bronchial biopsies were obtained from the carinae of second-order bronchi of the right, middle, and lower lobes.
For histologic analysis, all bronchoscopic biopsies were fixed in phosphate-buffered formalin, dehydrated in alcohol, and embedded in paraffin. Four-micrometer sections were cut from each tissue block, deparaffinized, dehydrated, and stained with hematoxylin and eosin (H&E). Subepithelial fibrosis was evidenced by the regular deposition of collagen fibers under the basement membrane. The measurements of subepithelial layer thicknesses were made from the base of the bronchial epithelium to its outer limit perpendicularly to the outermost edge of the basement membrane. The measurement, therefore, included the true basement membrane as well as reticular components. Maximum and minimum thicknesses were determined at each 3 different sites per biopsy sample.
For electron microscopy (EM), biopsy samples were fixed in 10% neutral formalin then in 3.0% glutaraldehyde, washed in cold 0.1 M phosphate buffer (pH 7.4), postfixed in 1% osmium tetroxide buffered with 0.1 M phosphate, and embedded in epoxy resin. Sections were cut using an ultramicrotome, stained with uranyl acetate and lead citrate, and examined under an EM (Jeol, Tokyo, Japan) (16). The areas of well-oriented, perpendicular, non-tangential epithelium and underlying RBM were photographed. The thickness of RBM was measured on photomicrographs.
Age, atopic index, FEV1, FVC, RBM, and subepithelial layer thickness results are expressed as means±SD. PC20 values are expressed as geometric means±SD. The Kruskal-Wallis and Mann-Whitney U tests were used for the analysis, and results with p values of <0.05 were considered significant. Data were analyzed using SPSS v12.0 (SPSS Inc., Chicago, IL, U.S.A.)
Atopic subjects with asymptomatic AHR were aged from 24 to 29 yr (mean, 25.6±2.1), non-atopic subjects with asymptomatic AHR 23-28 yr (mean, 25.4±2.5), atopic control groups 21-31 yr (mean, 27.0±4.8), non-atopic control groups 23-32 yr (mean, 26.8±3.4), and mild atopic asthmatics 21 or 22 yr. No statistical differences in age were found among these 5 groups (p>0.05, Table 1). Geometric means of PC20 were 2.58±0.53 in atopic subjects with asymptomatic AHR, 2.41±0.64 in nonatopic subjects with asymptomatic AHR, and 2.64±0.17 in mild atopic asthma patients, which were not statistically different (p>0.05, Table 1). Mean atopic indexes were 1.72±0.76 in atopic subjects with asymptomatic AHR, 1.94±0.54 in mild atopic asthma patients, and 1.83±1.17 in atopic controls (p>0.05, Table 1). In addition, no significant difference was observed among 5 groups with respect to basal FEV1 and FVC (p>0.05, Table 1).
On EM, mean RBM thicknesses were 4.3±1.7 µm in the mild atopic asthma group, 3.4±1.8 µm in the atopic asymptomatic AHR group, 2.5±1.5 µm in the non-atopic asymptomatic AHR group, 2.6±1.1 µm in the atopic controls, and 2.3±1.2 µm in the non-atopic controls (p=0.002, Fig. 1). Corresponding maximum RBM thicknesses were 5.5±1.6 µm, 4.5±1.8 µm, 3.5±1.3 µm, 3.4±0.8 µm, and 3.1±0.9 µm, respectively (p=0.002), and corresponding minimum RBM thicknesses were 3.0±0.7 µm, 2.3± 1.0 µm, 1.6±0.8 µm, 1.9±0.9 µm, and 1.4±0.7 µm, respectively (p=0.015). Mean RBM thicknesses were significantly higher in the mild atopic asthma group and the atopic asymptomatic AHR group than in the other three groups (p=0.048, Fig. 1, 2), which is also in minimum RBM thicknesses (p=0.037). Maximum RBM thicknesses showed a tendency to be higher in the atopic asymptomatic AHR group than in those of the non-atopic asymptomatic AHR group and of the control groups (p=0.06). No significant differences were observed between the atopic asymptomatic AHR group and mild atopic asthma group in terms of mean RBM thicknesses (p>0.05, Fig. 1), nor between the non-atopic asymptomatic AHR group and the two control groups in terms of mean RBM thicknesses (p>0.05, Fig. 1).
On light microscopy, mean subepithelial layer thicknesses were 6.1±3.1 µm in the mild atopic asthma group, 5.1±2.6 µm in the atopic asymptomatic AHR group, 3.9±1.8 µm in the non-atopic asymptomatic AHR group, 3.9±1.9 µm in the atopic control group, and 3.8±1.7 µm in the non-atopic control group (p=0.018, Fig. 3), and corresponding maximum subepithelial layer thicknesses were 8.4±2.7 µm, 7.1±2.0 µm, 5.4±1.0 µm, 5.6±1.1 µm, and 5.0±1.5 µm, respectively (p=0.002), and minimum subepithelial layer thicknesses were 3.8±1.5 µm, 3.1±0.9 µm, 2.3±0.5 µm, 2.5±0.6 µm, and 2.2±0.5 µm, respectively (p=0.001). The mean values of subepithelial layer thicknesses in the atopic asymptomatic AHR group were higher than in the non-atopic asymptomatic AHR group and in the two control groups (p=0.04, Fig. 3, 4), which is also in maximum, and minimum values of subepithelial layer thicknesses (p=0.005, p=0.008, respectively). No significant differences were observed between the atopic asymptomatic AHR and the mild atopic asthma groups in terms of mean subepithelial layer thicknesses (p>0.05, Fig. 3), nor between the non-atopic asymptomatic AHR group and the two control groups in terms of subepithelial layer thicknesses (p>0.05, Fig. 3).
In this study, RBM and subepithelial layer thickening was observed only in subjects with atopy among asymptomatic AHR subjects. This is in concordance with the findings of a previous asthma study (8), who reported that differences between atopic and nonatopic asthma subjects were found in terms of the degree of immunopathologic response. In that study, bronchial subepithelial tenascin and laminin layers were significantly thicker in the atopic group than in the nonatopic asthma group, and the amount of epithelial damage in the atopic asthma group was significantly greater than in control subjects or nonatopic asthmatics. Taken together, these findings suggest atopy may affect basement membrane thickness in asymptomatic AHR and in asthma.
A limited number of studies have examined bronchial inflammation and remodeling using bronchoscopic biopsies in asymptomatic AHR subjects. Laprise et al. found that asymptomatic AHR is associated with airway inflammation and remodeling, but did not subanalyze data with respect to atopic status (4). In that study, degrees of bronchial epithelial desquamation in asymptomatic AHR subjects were similar to those of asthmatic subjects, and CD3+, CD8+, and CD25+ T lymphocytes and eosinophils were elevated in the bronchial tissues of asymptomatic AHR subjects compared with in those of normal controls. However, they found that focal rather than continuous bronchial subepithelial fibrosis in asthmatics, and RBM thicknesses were not statistically different from those of normal controls. In our present study, we determined RBM maximum and minimum thicknesses at three different sites per bronchial biopsies. In terms of mean, minimum, and maximum values, RBM thicknesses were greater in asymptomatic AHR with atopy than in asymptomatic AHR without atopy or in normal controls. Moreover, these thicknesses were not focal but continuous, and were as great as observed in the two mild atopic asthmatic subjects.
Atopy and asymptomatic AHR may be independent risk factors of airway inflammation and remodeling. It has been suggested in familial studies of asthma and allergy that atopy and AHR are inherited independently (17, 18). The present study showed that RBM and subepithelial layer thicknesses were not elevated in non-atopic subjects with asymptomatic AHR or in atopic control subjects without asymptomatic AHR. In fact, RBM and subepithelial layer thicknesses were increased only in subjects with both atopy and asymptomatic AHR, indicating that atopy and asymptomatic AHR might additively or synergistically induce airway remodeling.
Asymptomatic AHR subjects may develop respiratory symptoms, and eventually asthma, more rapidly if they have atopy. Laprise et al. showed that four of 28 asymptomatic AHR subjects develop asthma symptoms after a 3-yr follow-up, and all four were sensitized to indoor allergens (4). Several reports have shown that the majority of subjects with AHR who eventually develop respiratory symptoms have a personal or family history of atopy (11, 17, 19-21). In the present study, one of seven subjects with atopic asymptomatic AHR developed asthma symptoms (exercise-induced wheezing and cough) after a 3-yr follow-up, which suggests that atopic asymptomatic AHR subjects may be more prone to develop asthma symptoms than non-atopic asymptomatic AHR subjects.
It has been reported that the RBMs are significantly thickened in rhinitis patients with or without asthma as compared with those of asymptomatic atopic or control subjects (6, 7). All of our study subjects denied rhinitis symptoms or other atopic diseases such as eczema. Thus, we are able to exclude the possibility that the RBM thickening observed in asymptomatic AHR subjects was a feature of atopic rhinitis.
The present study is the first to report that RBM and subepithelial layer are thickened in atopic and not in non-atopic subjects with asymptomatic AHR, and that this degree of thickening is comparable to that found in atopic asthma. This finding suggests that immunopathologic mechanisms differ in atopic and non-atopic asymptomatic AHR as in atopic and non-atopic asthma (8). In conclusion, we suggest that atopy may be an important determinant of subepithelial fibrosis in subjects with asymptomatic AHR.
Figures and Tables
Fig. 1
Reticular basement membrane (RBM) thicknesses. A, atopic asymptomatic AHR (n=5); B, non-atopic asymptomatic AHR (n=7); C, mild atopic asthma (n=2); D, atopic control (n=7); and E, non-atopic control (n=7). Horizontal bars within the graph indicate the mean values. The mean values of RBM thicknesses in A and C were significantly higher than in B, D, and E (p=0.048). No significant differences were observed between A and C or between B, D, and E in terms of mean RBM thickness.

Fig. 2
Bronchial histopathology (electron microscopy, ×3,750) in (A) atopic asymptomatic AHR, (B) non-atopic asymptomatic AHR, (C) mild atopic asthma, and (D) atopic control. RBM thicknesses showed greater in (A) and (C) than in (B) and (D).

Fig. 3
Subepithelial layer thicknesses. A, atopic asymptomatic AHR (n=5); B, non-atopic asymptomatic AHR (n=7); C, mild atopic asthma (n=2); D, atopic control (n=7); and E, non-atopic control (n=7). Horizontal bars within the graph indicate the mean values. The mean values of subepithelial layer thicknesses in A and C were significantly higher than in B, D, and E (p=0.04). No significant differences were observed between A and C or between B, D, and E in terms of mean subepithelial layer thickness.

Fig. 4
Bronchial histopathology (light microscopy, H&E staining, ×400) in (A) atopic asymptomatic AHR, (B) non-atopic asymptomatic AHR, (C) mild atopic asthma, and (D) atopic control. Subepithelial layer thicknesses showed greater in (A) and (C) than in (B) and (D).
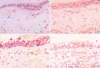
Table 1
Patient characteristics

Results were expressed as mean±SD for age, atopy index, FEV1, and FVC. *PC20 were expressed as geometric mean±SD. Atopic index was defined as the number of inhalant allergen categories (0-5) to which a patient showed at least one positive response on skin prick test. AHR, asymptomatic airway hyperreponsiveness; NS, not significant.
References
1. Boulet LP. Asymptomatic airway hyperresponsiveness: a curiosity or an opportunity to prevent asthma? Am J Respir Crit Care Med. 2003; 167:371–378.
2. Jansen DF, Timens W, Kraan J, Rijcken B, Postma DS. (A)symptomatic bronchial hyper-responsiveness and asthma. Respir Med. 1997; 91:121–134.

3. Kim YK, Kim SH, Tak YJ, Jee YK, Lee BJ, Kim SH, Park HW, Jung JW, Bahn JW, Chang YS, Choi DC, Chang SI, Min KU, Kim YY, Cho SH. High prevalence of current asthma and active smoking effect among the elderly. Clin Exp Allergy. 2002; 32:1706–1712.

4. Laprise C, Laviolette M, Boutet M, Boulet LP. Asymptomatic airway hyperresponsiveness: relationships with airway inflammation and remodelling. Eur Respir J. 1999; 14:63–73.

5. Boulet LP, Prince P, Turcotte H, Lemiere C, Olivenstein R, Laprise C, Larivee P, Begin P, Laviolette M. Clinical features and airway inflammation in mild asthma versus asymptomatic airway hyperresponsiveness. Respir Med. 2006; 100:292–299.

6. Braunstahl GJ, Fokkens WJ, Overbeek SE, KleinJan A, Hoogsteden HC, Prins JB. Mucosal and systemic inflammatory changes in allergic rhinitis and asthma: a comparison between upper and lower airways. Clin Exp Allergy. 2003; 33:579–587.

7. Milanese M, Crimi E, Scordamaglia A, Riccio A, Pellegrino R, Canonica GW, Brusasco V. On the functional consequences of bronchial basement membrane thickening. J Appl Physiol. 2001; 91:1035–1040.

8. Amin K, Ludviksdottir D, Janson C, Nettelbladt O, Bjornsson E, Roomans GM, Boman G, Seveus L, Venge P. Inflammation and structural changes in the airways of patients with atopic and nonatopic asthma. Am J Respir Crit Care Med. 2000; 162:2295–2301.

9. Laprise C, Boulet LP. Asymptomatic airway hyperresponsiveness: a three-year follow-up. Am J Respir Crit Care Med. 1997; 156:403–409.

10. Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, Sears MR. Outcome in adulthood of asymptomatic airway hyperresponsiveness in childhood: a longitudinal population study. Pediatr Pulmonol. 2002; 34:164–171.

11. Kerkhof M, Schouten JP, De Monchy JG. The association of sensitization to inhalant allergens with allergy symptoms: the influence of bronchial hyperresponsiveness and blood eosinophil count. Clin Exp Allergy. 2000; 30:1387–1394.

12. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987; 136:225–244.
13. Kim YK, Chang YS, Lee MH, Hong SC, Bae JM, Jee YK, Chun BR, Cho SH, Min KU, Kim YY. Role of environmental exposure to spider mites in the sensitization and the clinical manifestation of asthma and rhinitis in children and adolescents living in rural and urban areas. Clin Exp Allergy. 2002; 32:1305–1309.

14. Kim YK, Park HS, Kim HY, Jee YK, Son JW, Bae JM, Lee MH, Cho SH, Min KU, Kim YY. Citrus red mite (Panonychus citri) may be an important allergen in the development of asthma among exposed children. Clin Exp Allergy. 2001; 31:582–589.

15. American Thoracic Society. Medical section of the American lung association. Guidelines for fiberoptic bronchoscopy in adults. Am Rev Respir Dis. 1987; 136:1066.
16. Park SH, Kim MK, Kim H, Song BJ, Chi JG. Ultrastructural studies of gastrointestinal stromal tumors. J Korean Med Sci. 2004; 19:234–244.

17. Gray L, Peat JK, Belousova E, Xuan W, Woolcock AJ. Family patterns of asthma, atopy and airway hyperresponsiveness: an epidemiological study. Clin Exp Allergy. 2000; 30:393–399.

18. Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003; 111:215–225.
19. Rasmussen F, Lambrechtsen J, Siersted HC, Hansen HS, Hansen NC. Asymptomatic bronchial hyperresponsiveness to exercise in childhood and the development of asthma related symptoms in young adulthood: the odense schoolchild study. Thorax. 1999; 54:587–589.

20. Xuan W, Peat JK, Toelle BG, Marks GB, Berry G, Woolcock AJ. Lung function growth and its relation to airway hyperresponsiveness and recent wheeze. Results from a longitudinal population study. Am J Respir Crit Care Med. 2000; 161:1820–1824.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download