Abstract
Mycobacterium abscessus is the second most common etiology of pulmonary disease caused by nontuberculous mycobacteria in Korea. Although antimicrobial susceptibility tests are important for appropriate patient management in M. abscessus lung disease, the tests have never been investigated in Korea. Seventy-four isolates of M. abscessus recovered from patient respiratory samples were tested against eight antimicrobial agents following the guidelines set forth by the National Committee for Clinical Laboratory Standards. Of the parenteral antibiotics, amikacin (99%, 73/74) and cefoxitin (99%, 73/74) were active against most isolates. Imipenem (55%, 36/66) and tobramycin (36%, 27/74) had activity against moderate number of isolates. Of the oral antibiotics, clarithromycin (91%, 67/74) was active against the majority of isolates. Moxifloxacin (73%, 54/74) and ciprofloxacin (57%, 42/74) had activity against a moderate number of isolates. Doxycycline was the least active, inhibiting only 7% (5/74) of isolates. In conclusion, the variations in susceptibility within M. abscessus isolates to currently available antimicrobials suggest that the antimicrobial susceptibilities of any clinically significant M. abscessus isolate be needed individually.
Mycobacterium abscessus is the most pathogenic and chemotherapy-resistant rapidly growing mycobacteria (RGM). It accounts for 80% of lung disease caused by RGM and is the second most common RGM species present in extrapulmonary disease (following M. fortuitum) (1-4).
M. abscessus is resistant to all antituberculosis drugs including isoniazid, rifampin, ethambutol, and pyrazinamide. Thus, routine susceptibility testing to antituberculosis drugs is not recommended for this species. M. abscessus is considered to be susceptible to amikacin, cefoxitin, and clarithromycin, and moderately susceptible to imipenem (1-4). However, M. abscessus is not an easy organism to treat, and long-term treatment with multiple antimicrobial agents is usually required. Some studies have reported that in vitro susceptibilities to several antimicrobial agents are correlated with clinical responses to treatment in RGM infections (5, 6). Thus, drug susceptibility tests are important for appropriate patient management. In addition, all clinically significant isolates should be tested against selected antimicrobial agents (1, 2).
In Korea, M. abscessus is the second most common etiology of lung disease caused by nontuberculous mycobacteria (NTM) (following the M. avium-intracellulare complex). It accounts for 21 to 33% of NTM lung disease in Korea (7-9); however, in vitro antimicrobial susceptibility test results for this organism have never been investigated in Korean population. Therefore, we examined the in vitro susceptibilities of M. abscessus isolates from patient respiratory specimens that displayed M. abscessus lung disease.
From July 2005 to December 2006, 74 nonduplicate isolates of M. abscessus (one isolate per patient) recovered from respiratory clinical samples were collected for use in this study. NTM species identification was performed using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method based on the rpoB gene (10). All patients met the diagnostic criteria recommended by the American Thoracic Society for M. abscessus lung disease (11).
Antimicrobial susceptibility testing was performed at the Korean Institute of Tuberculosis according to the guidelines set forth by the National Committee for Clinical Laboratory Standards (NCCLS) (12, 13). We tested eight antimicrobial agents against M. abscessus isolates: amikacin (Sigma, St. Louis, MO, U.S.A.), cefoxitin (Sigma), imipenem (MSD, Seoul, Korea), tobramycin (Sigma), clarithromycin (Hanmi, Seoul, Korea), ciprofloxacin (Ildong, Seoul, Korea), moxifloxacin (Bayer, Seoul, Korea), and doxycycline (Sigma). Minimal inhibitory concentrations (MICs) of all tested drugs were determined by the broth microdilution method and interpreted according to the guidelines described by the NCCLS document M24-A in 2003 (12). The MIC of moxifloxacin for this RGM has not yet been described by the NCCLS. In its place, we followed the recommendation for aerobic organisms in NCCLS M100-S11 in 2001 (13).
The colonies present on the culture medium were transferred to 15-mL tubes of 7H9 broth (Becton Dickinson, Piscataway, NJ, U.S.A.) with 0.02% Tween 80 (Junsei Chemical, Tokyo, Japan). The suspension was mixed vigorously on a vortex mixer for 15 to 20 sec until the turbidity matched the density of a 0.5 McFarland standard. The suspension was then diluted a few times following consideration of the final inoculum concentrations in the well. Serial 2-fold dilutions of antimicrobial agents were prepared and added across the 96-well plates (Becton Dickinson). The concentrations of antimicrobial agents in the wells were determined based on the NCCLS recommendation (12, 13). The final inoculum suspension (150 µL) was transferred to the wells of a microtiter tray containing 150 µL of each antimicrobial type and dilution. The final inoculum concentration was 5×104 CFU/mL per well. The inoculated tray was covered and incubated at 30℃ for over 3 days in room air. Endpoint MICs of all the antimicrobial drugs were read as the first well in which no growth occurred. Staphylococcus aureus ATCC 29213 and Mycobacterium peregrinum ATCC 700686 were also used for quality control purposes. The susceptibility categories of all antimicrobial agents were determined according to the breakpoints recommended by the NCCLS and are presented in Table 1 (12, 13).
In total, 74 isolates of M. abscessus were tested for antimicrobial susceptibility. The results of antimicrobial susceptibility tests are presented in Table 2. Of the parenteral antibiotics, amikacin (99%, 73/74) and cefoxitin (99%, 73/74) were active against most M. abscessus isolates. Amikacin demonstrated better in vitro activity against M. abscessus than tobramycin (36%, 27/74). Imipenem (55%, 36/74) had in vitro activity against a moderate number of isolates. Of the oral antibiotics, clarithromycin (91%, 67/74) was active against the majority of isolates. The fluoroquinolones showed moderate in vitro activities against the M. abscessus isolates. Moxifloxacin (73%, 54/74) had better in vitro activity than ciprofloxacin (57%, 42/74). Doxycycline was the least active, inhibiting only 7% (5/74) of the M. abscessus isolates.
M. abscessus isolates have been determined to be uniformly resistant to standard antituberculous agents. For pulmonary diseases caused by M. abscessus, it is recommended that susceptible oral antibiotics (including clarithromycin or azithromycin) be administered in combination with parenteral medications (amikacin, cefoxitin, or imipenem) (1, 2). Due to its variable in vitro susceptibilities to some drugs, antibiotic susceptibility testing of all clinically significant isolates is recommended, although the correlation between in vitro susceptibility results for M. abscessus and clinical response for specific antimicrobial agents has not been established (1, 2). Our results in this study demonstrated that the resistance rates of M. abscessus isolates to various antibiotics are high. However, amikacin and cefoxitin were active against nearly all M. abscessus isolates. Clarithromycin was generally active against the majority of the M. abscessus isolates. In addition, ciprofoxacin, moxifloxacin, and imipenem exhibited moderate activity (>50% susceptibility), but the susceptibility of M. abscessus to doxycyline was poor.
Aminoglycosides are important parenteral antibiotics used in the treatment of M. abscessus infection. Traditionally, amikacin was the most active agent against RGM species. According to a study by Swenson et al., 95% of M. abscessus, 99% of M. fortuitum, and 88% of M. chelonae isolates were inhibited by this agent with a MIC of ≤16 µg/mL (5). Intravenous amikacin is administered at a dose of 10 to 15 mg/kg daily to adult patients with normal renal function. This dose elicits peak serum levels in the low 20 mg/mL range. In this study, 99% of M. abscessus isolates were susceptible to amikacin and 36% were susceptible to tobramycin.
Cefoxitin is another important parenteral antibiotic used in the treatment of M. abscessus infection. This agent is administered at a dose of up to 12 g/day intravenously in divided doses. In this study, cefoxitin inhibited the growth of 99% of M. abscessus isolates. Another parenteral antibiotic that has been used in M. abscessus infection is imipenem. This agent has been reported to be moderately susceptible against M. abscessus (14). This study demonstrated that imipenem inhibits 55% of M. abscessus isolates at the breakpoint of <8 µg/mL and 88% of isolates at the intermediate breakpoint of <16 µg/mL. Therefore, imipenem may be useful in clinical treatment regimens for M. abscessus, especially in situations when cefoxitin cannot be used due to adverse effects.
Of the variable oral antibiotics, the most effective agents are the newer macrolides. Clarithromycin is representative of this class and has been considered to be the most effective (12). In this study, 91% of M. abscessus isolates were susceptible and 3% were resistant to clarithromycin. Brown et al. reported that the newer macrolides generally show better activity against RGM species, and among them, clarithromycin is the strongest (15).
Oral fluoroquinolones have also been used in the treatment of RGM diseases. Ciprofloxacin is the class representative of the older fluoroquinolones (i.e., ciprofloxacin, ofloxacin, and levofloxacin). In the current study, we tested ciprofloxacin and moxifloxacin (newer 8-methoxyfluoroquinolone), and both antimicrobials showed high activities, with 57 and 73%, respectively, of M. abscessus isolates susceptible. Even though fluoroquinolone cannot be used as a single-drug therapy due to the risk of developing mutational resistance (16), both ciprofloxacin and moxifloxacin could be used as alternative oral agents during combination treatment for M. abscessus lung disease. Doxycycline is a member of the tetracycline antibiotics and has shown poor activity against M. abscessus and M. chelonae in previous studies. In the current study, only 21% of M. abscessus isolates were susceptible or intermediate and approximately 80% were resistant to this agent. Doxycycline was the least active agent among all those tested in this study.
In this study, NTM species identification was performed using the PCR-RFLP method based on the rpoB gene. RGM are difficult to speciate by conventional methods. Several genes such as 16S rRNA, hsp65, and rpoB gene have been used to identify NTM to the species level. However, some gene target is often limited by the lack of sequence divergence among closely related Mycobacterium species. For example, 16S rRNA internal transcribed spacer assay cannot differentiate M. abscessus from M. massiliense or M. bolletii, although this assay has proven to be valuable to distinguish M. abscessus and M. chelonae (17). Accurate identification of isolated NTM species is important since distinguishing these species is clinically relevant.
In conclusion, the variations in susceptibility within M. abscessus isolates to currently available antimicrobials suggest that the antimicrobial susceptibility testing of all clinically significant M. abscessus isolates be needed. In addition, our results offer clinicians choices for empirical treatment when M. abscessus lung disease is diagnosed and the in vitro susceptibilities are not available.
Figures and Tables
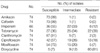
Table 1
Antimicrobial drugs and MIC breakpoints

MIC, minimal inhibitory concentration.
Drugs and breakpoints are listed according to the recommendations set forth by the NCCLS document M24-A (12).
*The breakpoints of this antimicrobial agent for the RGM have not yet been addressed by the NCCLS. The values are those recommended for aerobic organisms in NCCLS M100-S11 (13).
References
1. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007. 175:367–416.

2. Daley CL, Griffith DE. Pulmonary disease caused by rapidly growing mycobacteria. Clin Chest Med. 2002. 23:623–632.

3. Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993. 147:1271–1278.

4. Wallace RJ Jr, Swenson JM, Silcox VA, Good RC, Tschen JA, Stone MS. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis. 1983. 5:657–679.

5. Swenson JM, Wallace RJ Jr, Silcox VA, Thornsberry C. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985. 28:807–811.

6. Wallace RJ Jr, Swenson JM, Silcox VA, Bullen MG. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis. 1985. 152:500–514.

7. Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, Bai GH. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006. 129:341–348.

8. Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci. 2005. 20:913–925.

9. Yim JJ, Park YK, Lew WJ, Bai GH, Han SK, Shim YS. Mycobacterium kansasii pulmonary diseases in Korea. J Korean Med Sci. 2005. 20:957–960.

10. Lee H, Park HJ, Cho SN, Bai GH, Kim SJ. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J Clin Microbiol. 2000. 38:2966–2971.
11. Wallace RJ Jr, Cook JL, Glassroth J, Griffith DE, Olivier KN, Gordin F. American Thoracic Society statement: diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med. 1997. 156:S1–S25.
12. National Committee for Clinical Laboratory Standards. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; Approved Standard. 2003. Wayne, PA: NCCLS;Document No. M24-A.
13. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Eleventh informational supplement. 2001. Wayne, PA: NCCLS;Document No. M100-S11.
14. Wallace RJ Jr, Brown BA, Onyi GO. Susceptibilities of Mycobacterium fortuitum biovar. fortuitum and the two subgroups of Mycobacterium chelonae to imipenem, cefmetazole, cefoxitin, and amoxicillin-clavulanic acid. Antimicrob Agents Chemother. 1991. 35:773–775.

15. Brown BA, Wallace RJ Jr, Onyi GO, De Rosas V, Wallace RJ 3rd. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and M. chelonae-like organisms. Antimicrob Agents Chemother. 1992. 36:180–184.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download