Abstract
Mesenchymal stem cells (MSCs) have recently been identified and characterized in humans. Moreover, MSC secrete cytokines that can support hematopoietic progenitor growth. In the present study, we evaluated whether the efficacy of hematopoietic stem cell transplantation is improved by their co-transplantation with MSC, and whether this is positively correlated with the dose of infused MSCs. Accordingly, irradiated NOD/SCID mice were transplanted with 1×105 human CD34+ cells in the presence or absence of culture expanded MSCs (1×106 or 5×106). We evaluated human hematopoietic cell engraftment by flow cytometry and assessed MSC tissue distributions by fluorescence in situ hybridization. We found that CD45+ and CD34+ cell levels were significantly elevated in a dose-dependent manner in cotransplanted mice 4 weeks after transplantation. The engraftments of CD33+ and CD19+ cells also increased dose-dependently. However, the engraftment of CD3+ cells did not increase after co-transplantation with MSCs. Human Y chromosome+ cells were observed in multiple tissues and were more frequently observed in mice co-transplanted with 5×106 rather than 1×106 MSCs. These results suggest that MSCs are capable of enhancing hematopoietic cell engraftment and distribution in multiple organs in a dose-dependent fashion.
In addition to hematopoietic stem cells (HSCs), the bone marrow contains a second type of stem cells that are capable of giving rise to multiple mesenchymal cell lineages, namely, mesenchymal stem cells (MSCs) (1, 2). Bone marrow stromal cells have been shown to include MSCs, which are thought to support hematopoiesis by creating an optimal microenvironment (the marrow matrix), and by providing cytokines and other regulatory factors that stimulate and enhance proliferation of the hematopoietic elements (3). Moreover, multipotent human MSCs can be isolated from bone marrow and expanded more than 1-billion-fold in cell culture without loss of stem cell capacity (4). These cells are capable of differentiating into osteoblasts, adipocytes, chondrocytes, myocytes, neural elements, and stromal fibroblasts under defined in vitro or in vivo conditions, and show an identical phenotype when grown from a single cell clone (5-7). In the area of hematopoiesis, MSCs are being used clinically to support hematopoietic recovery by replacing stroma. Moreover, a growing body of clinical evidence suggests the existence of a link between the efficiency of hematopoietic recovery and the degree of stromal damage that occurs as a result of myeloablative cancer therapy (8). As a result, therapy-ablated marrow may not be able to sustain hematopoietic stem cell maintenance or their differentiation into specific lineages, such as, megakaryocytes and platelets. In addition, a prolonged stromal defect in growth factor production, like early-stage cytokines, such as, interleukin-6, leukemia inhibitory factor, stem cell factor and granulocyte-colony stimulating factor, after autologous bone marrow transplantation has been observed for as long as a year in patients with hematologic disorders (9).
Independent laboratories have shown that recipients of unmanipulated allogeneic bone marrow transplants have only host-type marrow stromal cells and MSCs in bone marrow (10, 11). These results are attributed to the inability of the conditioning regimen to ablate host marrow stroma and/or the inability of stromal progenitors of donor origin to engraft. In addition, the number of MSCs has been estimated to be low in an average bone marrow graft (400-1,000 MSCs/kg). On the other hand, a recent report noted that allogeneic osteoblasts could be detected in recipients with osteogenesis imperfecta (OI) after sibling-matched allogeneic bone marrow transplantation (12). These findings suggest that osteoblasts may be transplanted successfully under certain conditions. Stromal chimerism has been achieved using infusions of cultureexpanded stromal progenitors in murine models, and xenotransplantation models have been developed to determine the homing of human MSCs in animals. Human MSCs were detected predominantly in bone marrow, and less frequently in other organs of immunodeficient NOD/SCID mice 8 weeks after infusion. Moreover, recent data indicate that MSC coinfusion enhances human hematopoiesis in NOD/SCID mice (13, 14). Based on encouraging data obtained in both rodent and large animal models, clinical studies of MSC transplantation are underway in both autologous and allogeneic settings. Koc et al. (15) found that the co-infusion of autologous MSCs together with autologous HSCs is free of toxicity and that it is associated with rapid hematopoietic recovery in 28 women receiving high dose chemotherapy for advanced breast carcinoma. In this report, we demonstrate that co-transplantation with human MSCs can enhance the engraftment of human hematopoietic stem cells in NOD/SCID mice, and that this engraftment-enhancing effect increases according to the number of infused MSCs. We also show that an increased dose of MSCs enhances the tissue distribution of human MSCs in multiple tissues in NOD/SCID mice. Our results show that MSCs are capable of enhancing hematopoietic engraftment and distribution to multiple organs in a dose-dependent fashion.
UCB was obtained at the time of full-term deliveries, after receiving informed consent for the study protocol by our hospital's ethics committee. UCB was collected after clamping and cutting of cords by draining blood into sterile collection tubes containing the anticoagulant citrate-phosphate dextrose. Mononuclear cells (MNCs) were isolated from UCB using Ficoll-Hypaque (1.077 g/cm3, Sigma, St Louis, MO) density centrifugation (400 g for 25 min). CD34+ cells were isolated using a magnetic cell sorting system, Mini-MACS (Miltenyi Biotec, Auburn, CA), according to the manufacturer's instructions. Purity was determined by flow cytometry using a FITC-conjugated anti-CD34 (anti-HPCA-2, Becton-Dickinson, Mountain View, CA), and was found to be 90 to 95%.
Ten to twenty mililiters of bone marrow aspirate was obtained under sterile conditions by posterior iliac crest puncture from bone marrow transplantation donors with given informed consent. Human MSCs were isolated and culture-expanded according to the method described by Pittenger et al. (1). MNCs were isolated from bone marrow, and cells were cultured in human MSC medium composed of Dulbecco's modified Eagle's low glucose medium (DMEM-LG; GibcoBRL, Grand Island, NY) containing 10% fetal bovine serum (FBS; GibcoBRL) and 1% antibiotic-antimycotic solution (GibcoBRL). Human MSCs were confirmed to be negative for hematopoietic markers by flow cytometry and to be capable of differentiating into osteocytes, chondrocytes, and adipocytes in vitro (Fig. 1, 2).
MSCs were induced to differentiate into adipocytes by treating confluent second passage monolayer cultures with 1 µM dexamethasone (Sigma, St. Louis, U.S.A.), 0.5 mM methylisobutylxanthine (Sigma), 10 µg/mL insulin (Sigma), and 100 mM indomethacin (Sigma) in DMEM containing 10% FBS. Adipogenic differentiation was demonstrated by the accumulation of lipid vesicles stained red by Oil red O (Sigma). The osteogenic differentiation of MSCs was induced by culturing confluent monolayers in DMEM containing 10% FBS, 0.1 µM dexamethasone, 50 µM ascorbate (Sigma), and 10 µM β-glycerol- phosphate (Sigma). MSC osteogenic differentiation over 21 days was demonstrated by increases in alkaline phosphatase and calcium accumulation. Alkaline phosphatase was detected histologically, and calcium was stained by the Kossa's method. The chondrogenic differentiation of MSCs was in- duced by pellet culture in 500 µL of DMEM containing 10% FBS, 0.1 µM dexamethasone, 50 µM ascorbic acid 2-phosphate (Sigma), and 1 µg/mL transforming growth factor-β (βTGF-β R&D System, Minneapolis, MN). Under low speed centrifugation (1,000 g for 5 min), a dense mass of cells formed at the bottom of conical centrifuge tubes. Chondrogenic differentiation was demonstrated by toluidine blue staining after 3 weeks of culture.
Female NOD/SCID mice (5-6 weeks old), purchased from Charles-River Laboratory (Tokyo), were housed in micro-isolator cages on laminar flow racks at the animal facilities of the Catholic University of Korea. Mice were provided a sterile diet and autoclaved acidified water.
NOD/SCID mice were sublethally irradiated (3.5 Gy) 4 to 6 hr before transplantation. Human female cord blood CD34+ cells were injected at a final volume of 100 µL Iscove's Modified Dulbecco's Media (IMDM; GibcoBRL) per mouse and human male MSCs were injected with IMDM at a volume of 100 µL/106 MSCs per mouse. Both CD34+ cells and MSCs were injected via a tail vein. Two mice were assigned to each experimental group. In three separate experiments, mice were assigned to receive 1×105 CD34+ cells, CD34+ cells plus 1×106 MSCs, or CD34+ cells plus 5×106 MSCs. One mouse treated with 5×106 MSCs in the first experiment died after MSCs infusion due to myocardial infarction (pathologically confirmed, data not shown).
Four weeks after transplantation, mice were sacrificed by carbon dioxide inhalation, and bone marrow (BM) cells were collected by flushing both femurs with RPMI medium (Gibco BRL). Cell suspensions were stained with mouse anti-human monoclonal antibodies, fluorescein isothiocyanate (FITC)-conjugated CD45 antibody and phycoerythrin (PE)-conjugated CD34, CD13, CD33, CD3, and CD19 antibodies (Becton-Dickinson, Mountain View, CA). Flow cytometry to detect human CD45+ and CD34+, CD13+, CD33+, CD3+, or CD19+ cells was performed on a FACScan flow cytometer (Becton-Dickinson). FITC- and PE-conjugated mouse isotype control antibodies were used for each culture, and 5,000-10,000 events were counted for each analysis. Differences in engraftment percentages were calculated using the Student's t-test. p values of <0.05 were considered statistically significant.
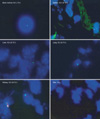
Fluorescence in situ hybridization (FISH) for the human Y chromosome was used to detect transplanted MSCs in BM, spleen, liver, lung, kidney, heart, skeletal muscle, intestine, and skin of NOD/SCID mice at four weeks after transplantation in the second and third experiments. Total numbers of Y chromosome positive cells in ten×high-power fields were counted.
Culture of bone marrow MNCs produced a monomorphic confluent adherent layer of elongated fibroblast-like cells that survived multiple passages under mesenchymal culture conditions. At the end of the second passage, bone marrow derived MSCs had successfully differentiated along osteogenic, chondrogenic, and adipogenic lineages, using methods described above (Fig. 2).
Transplantation of UCB CD34+ cells (1×105/mouse) in the presence of MSCs (1×106 and 5×106/mouse) resulted in significantly higher engraftment levels in the BM of NOD/SCID mice than was observed after transplanting UCB CD34+ cells alone. The mean percentage of CD45+ cells in the BM of NOD/SCID mice according to the MSCs number, i.e., 1×106 or 5×106, increased to 41.05±5.62 and 55.58±8.26, respectively, compared with 29.18±1.00 for transplantation with CD34+ cells without MSCs (p<0.05). The difference between the group infused with 1×106 MSCs and the group infused with 5×106 MSCs was statistically significant (p<0.05) (Fig. 3). The mean percentage of CD45+-CD34+ cells increased to 13.78±1.50 and 17.43±2.05 at these MSC dosages, compared with 7.76±0.37 for transplantation with CD34+ cells without MSCs (p<0.05). In addition, the groups infused with 1×106 or 5×106 were significantly different (p<0.05) (Fig. 3).
Co-transplantation of MSCs significantly increased the level of human myeloid cell engraftment in NOD/SCID mouse BM. The mean percentage of CD45+-CD33+ cells in NOD/SCID mouse BM at MSC doses of 1×106 and 5×106 were 19.62±1.18 and 24.38±3.05, respectively, compared with 6.54±0.17 for transplantation with CD34+ cells alone (p< 0.05). Moreover, the level of myeloid cell engraftment in the group infused with a high dose of MSCs was significant higher than in the group infused with a lower dose (p<0.05) (Fig. 3). Mean percentages of CD45+-CD13+ cells increased to 21.64±2.04 and 24.45±3.41 for high and low MSC doses, compared with 10.34±0.57 for transplantation with CD34+ cells alone (Fig. 3). Although there was a distinct difference between the group co-transplanted with MSCs and the group transplanted with HSC alone (p<0.05), we did not notice a difference between levels of HSC engraftment according to the number of MSCs co-transplanted.
The mean percentages of CD45+-CD19+ cells in NOD/SCID mouse BM according to the MSC dose were significantly increased to 9.93±4.77 and 24.39±11.22, respectively, compared with 7.08±1.46 of CD34+ cells alone (p<0.05). Co-transplantation of MSCs at 5×106 significantly increased the level of human B lymphoid cell engraftment in NOD/SCID mouse BM (p<0.05). Mean percentages of CD45+-CD3+ cells engrafted by low and high MSC doses were 2.89±1.90 and 4.43±1.64, respectively, compared with 3.02±1.17 for CD34+ cells alone. Co-transplantation of MSCs did not increase the level of human T lymphoid cell engraftment in NOD/SCID mouse BM (Fig. 3).
Human Y chromosome positive cells were found in BM, spleen, liver, lung, kidney, heart, intestine, and skin of NOD/SCID mice at four weeks after transplantation but not in skeletal muscle. The distribution of human Y chromosome positive cells differed by tissue; Y chromosome positive cells were found more frequently in the liver, lung, and kidney than in other tissues (Fig. 4, Table 1). In addition, numbers of these cells were higher in mice that received a higher dose of MSCs in same tissues.
We infused relatively low doses of CD34+ cells (1×105 cells) into NOD/SCID mice in this study. We tried to evaluate the dose-dependent effect of MSCs on HSC engraftment, and thus, we used relatively high doses of MSCs (5×106/animal) as compared with the doses (1×106/animal) usually applied. We observed that the high-dose MSC group showed significantly higher levels of HSC engraftment than the lowdose (1×106/animal) MSC group, thus confirming that MSCs have a dose-dependent effect on HSC engraftment.
Noort et al. (13) demonstrated in NOD/SCID mice that engraftment levels were higher in the co-transplantation of MSCs (1×106) and UCB CD34+ cells than in transplantation with UCB CD34+ cells alone. They also demonstrated that the effects of MSC co-transplantation on BM engraftment were more pronounced after transplantation with relatively low doses of CD34+ cells (0.03-0.1×106). The engraftment level increased three- to four-fold with MSCs cotransplantation with low doses of UCB CD34+ cells. However, no further additive effect was observed at higher doses of UCB CD34+ cells. Angelopoulos et al. (14) reported that MSC co-transplantation enhanced human cell engraftment when CD34+ cell infusion was limited, and that the efficacy of engraftment was blunted when the CD34+ cell dose was increased to 1.5×106. These data suggested that MSC co-transplantation can enhance the efficacy of engraftment if only a low dose of CD34+ cells is available. We observed similar effects in the present study.
Few reports have evaluated whether the engraftment-enhancing effect of MSCs is related to the MSC dose, which is why we decided to use a higher dose in this study. However, problems occur when animal models are administered higher doses. Initially, we wondered whether higher doses increase the risk of lethal vascular complications, such as, pulmonary embolism. In fact, the frequency of sudden death within 24 hr after MSC infusion did tend to increase slightly, but this was not significant (not shown data). Additionally, the use of a high cell dose could be criticized for not being clinically relevant. However, the objective of this study was to determine the effect that MSCs have on HSC engraftment, and therefore we chose a somewhat extreme model.
The effect of MSCs on HSC engraftment is not lineage-restricted, with grafts being comprised B lymphoid cells as well as myeloid cells. However, T lymphoid cell levels were not increased by co-transplanting HSCs with MSCs. This supportive activity may have been derived from the specific characteristics of MSCs, which have a suppressive effect on the proliferation of T lymphocytes and a modulating effect on the immune system (16, 17). However, no mechanism explaining the immunoregulatory effects of MSCs has been elucidated (16-20).
Several studies in animal models have demonstrated that stromal cells not only seed the bone marrow but also enhance hematopoietic recovery (13, 21, 22). However, the transplantation ability of bone marrow stromal elements still remains an open issue in humans (23). Several independent clinical studies have shown that recipients transplanted with unmanipulated allogeneic bone marrow have only host-type marrow stromal cells and MSCs in bone marrow (11, 24). This finding suggests that neither stromal progenitors nor MSCs are able to engraft into bone marrow because a "normal" bone marrow graft contains too few MSCs to support engraftment. However, others have reported contrary results (25, 26). This discordance may originate from either methodological differences concerning the detection of donor-derived mesenchymal cells, or from differences between doses of reinfused stromal cells. To detect engrafted donor derived cells in various tissue types, green fluorescence protein (GFP) is most widely used as a reporter. However, even though the GFP method has a higher sensitivity than the monoclonal antibody method for membrane antigen, it is difficult to determine whether GFP expressing cells are quiescent. Therefore, we decided to use FISH for the human X and Y chromosomes to detect engrafted donor derived cells in the organs of NOD/SCID mice. The data obtained provide evidence that human HSCs can be engrafted into various organs in NOD/SCID mice, and that the number of engrafted cells increases in proportion to the number of MSCs infused.
Our results reveal that MSCs have an accelerating effect on HSC engraftment and that this is dose-dependent. It is also suggested that a graft engineered to increase the number of MSCs may pave the way for further applications in the field of transplantation with respect to hematopoietic support and efficient engraftment.
Figures and Tables
Fig. 1
Immunophenotype of cultured human mesenchymal stem cells. Mesenchymal stem cells expressed CD105 and CD166, but were negative for CD34, CD45, and CD14.

Fig. 2
Culture-expanded human mesenchymal stem cells exhibited a fibroblastic morphology (top panel). Under specific differentiation-inducing conditions, mesenchymal stem cells differentiated into osteoblasts, chondrocytes, and adipocytes.
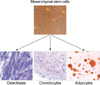
Fig. 3
Effects of MSCs on human UCB 34+ cell engraftment in NOD/SCID mice. Co-transplantation with UCB CD34+ cells and MSCs resulted in higher engraftment levels in NOD/SCID mouse bone marrow 4 weeks after transplantation than after transplantation with UCB CD34+ cells alone. Moreover, this engraftment-promoting effect was related to the MSC dosage and increased myeloid and B lymphoid cell numbers but not those of T-lymphoid cells. *A significant difference (p<0.05) between the cotransplanted group and the group transplanted with CD34+ alone. †Significant (p<0.05) between the group infused with 1×106 MSCs and the group infused with 5×106 MSCs.

Fig. 4
Human X chromosome (green) and Y chromosome (red) expression by fluorescence in situ hybridization (FISH) analysis. Human X and Y chromosomes were detected in bone marrow, spleen, liver, lung, and kidney. Y chromosomes were detected in the heart, intestine, and skin (results of heart and intestine are not shown).
Notes
References
1. Pitterger MK, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
2. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418:41–49.

3. Deans RJ, Moseley AB. Mesenchymal stem cells; biology and potential clinical uses. Exp Hematol. 2000. 28:875–884.
4. Koc ON, Lazarus HM. Mesenchymal stem cells: heading into clinic. Bone Marrow Transplant. 2001. 27:235–239.
5. Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotype and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998. 176:57–66.
6. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–74.

7. Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AB, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000. 6:1282–1286.

8. Galotto M, Berisso G, Delfino L, Podesta M, Ottaggio L, Dallorso S, Dudour C, Ferrara GB, Abbondandolo A, Dini G, Bacigalupo A, Cancedda R, Quarto R. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol. 1999. 27:1460–1466.

9. Roingeard F, Binet C, Lecron JC, Truglio D, Colombat P, Domenech J. Cytokines released in vitro by stromal cells from autologous bone marrow transplant patients with lymphoid malignancy. Eur J Haematol. 1998. 61:100–108.

10. Simmons PJ, Przepiorka D, Thomas ED, Torok-Storb B. Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature. 1987. 328:429–432.

11. Koc ON, Peters C, Aubourg P, Raghavan S, Dyhouse S, DeGasperi R, Kolodny EH, Yoseph YB, Gerson SL, Lazarus HM, Caplan AI, Watkins PA, Krivit W. Bone marrow-derived mesenchymal stem cells remain host-derived despite successful hematopoietic engraftment after allogeneic transplantation in patients with lysosomal and peroxisomal storage diseases. Exp Hematol. 1999. 27:1675–1681.
12. Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999. 5:309–313.
13. Noort WA, Kruisselbrink AB, in 't Anker PS, Kruger M, van Bezooijen RL, de Paus RA, Heemskerk MH, Lowik CW, Falkenburg JH, Willemze R, Fibbe WE. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34+ cells in NOD SCID mice. Exp Hematol. 2002. 30:870–878.
14. Angelopoulou M, Novelli E, Grove JE, Rinder HM, Civin C, Cheng L, Krause DS. Co-transplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytosis in NOD/SCID mice. Exp Hematol. 2003. 31:413–420.
15. Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000. 18:307–316.
16. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002. 30:42–48.

17. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or non-specific mitogenic stimuli. Blood. 2002. 99:3838–3843.

18. Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003. 57:11–20.

19. McIntosh K, Bartholomew A. Stromal cell modulation of the immune system: a potential role for mesenchymal stem cells. Graft. 2000. 3:324–328.
20. Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003. 101:3722–3729.

21. in 't Anker PS, Noort WA, Kruisselbrink AB, Scherjon SA, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE. Nonexpanded primary lung and bone marrow-derived mesenchymal cells promote the engraftment of umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2003. 31:881–889.
22. Thalmeier K, Huss R. Highly efficient retroviral gene transfer into immortalized CD34(-) cells and organ distribution after transplantation into NOD/SCID mice. Cytotherapy. 2001. 3:245–251.

24. Cilloni D, Carlo-Stella C, Falzetti F, Sammarelli G, Regazzi E, Colla S, Rizzoli V, Aversa F, Martelli MF, Tabilio A. Limited engraftment capacity of bone marrow-derived mesenchymal cells following T-cell-depleted hematopoietic stem cell transplantation. Blood. 2000. 96:3637–3643.

25. Fouillard L, Bensidhoum M, Bories D, Bonte H, Lopez M, Moseley AM, Smith A, Lesage S, Beaujean F, Thierry D, Gourmelon P, Najman A, Gorin NC. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia. 2003. 17:474–476.

26. Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, Sturgeon C, Hewett T, Chung T, Stock W, Sher D, Weissman S, Ferrer K, Mosca J, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001. 29:244–255.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download