Abstract
Allergic bronchopulmonary aspergillosis (ABPA), an asthmatic disease, is caused primarily by hypersensitivity to Aspergillus species. ABPA is rarely observed in the absence of asthma, which is, in fact, the principle criterion for its diagnosis. Here, we report the case of a 36-yr-old woman without a history of bronchial asthma, who manifested a localized pneumonic consolidation, coupled with broncholithiasis. Pathologic examinations of bronchoscopic biopsy specimens and resected surgical specimens revealed features typical of ABPA. This is a very rare case of ABPA coupled with broncholithiasis in a non-asthmatic individual.
Allergic bronchopulmonary aspergillosis (ABPA) is a complex hypersensitivity reaction, which occurs predominantly in asthmatic patients and patients with cystic fibrosis. ABPA has been estimated to occur in 1-2% of chronic asthmatics (1) and in up to 10% of cystic fibrosis patients (2). Its clinical and diagnostic manifestations originate from an allergic response to multiple antigens expressed by fungi, most notable among which is Aspergillus fumigatus, which can colonize the bronchial mucus (3). Repeated episodes of bronchial obstruction, inflammation, and mucoid impaction can result in bronchiectasis, fibrosis, and respiratory compromise.
ABPA is rarely observed in the absence of asthma, and asthma constitutes the principle criterion for the diagnosis of ABPA. In addition, ABPA is radiologically characterized by the presence of bronchiectasis, which is generally central and involves at least three lobes (4, 5). Here, we report a case of a 36-yr-old woman who did not exhibit clinical asthma but manifested a localized pneumonic consolidation coupled with broncholithiasis. The patient was ultimately diagnosed with ABPA, after a series of laboratory investigations and a surgical lung resection.
A 36-yr-old woman was referred to the outpatient chest clinic at Samsung Medical Center for evaluation of a chronic cough which had persisted for two months with minimal expectoration, coupled with an intermittent low-grade fever and myalgia. The patient reported no history of wheezing or dyspnea, nor did she have any personal or family history of atopy. The patient did not smoke, and had experienced no previous diseases of this nature, such as bronchial asthma or pulmonary tuberculosis.
The patient had a total leukocyte count of 8,300 cells/µL with 12% eosinophils (eosinophil count of 1,000 cells/µL). Her serum total IgE level was 488 U/mL. Routine skin prick tests were performed with 12 common inhalant allergens (Allergopharma Joachim Ganzer KG, Reinbek, Germany). The mean diameter of the wheal of the Aspergillus fumigatus and the histamine was 3 mm and 3.5 mm, respectively. Skin reactivity to other allergens was negative. Her sputum cultures, however, yielded no Aspergillus species growth. Specific IgE against A. fumigatus measured using the CAP system was positive (1.17 kU/L), and precipitating antibodies against A. fumigatus measured using immunodiffusion method were positive. Spirometry was normal, with a forced vital capacity (FVC) of 2.79 L (82% of predicted), a forced expiratory volume in 1 sec (FEV1) of 2.27 L (85% of predicted), and an FEV1/FVC ratio of 81%. The methacholine inhalation test revealed no bronchial hyperreactivity.
The patient's chest radiography revealed a mass-like segmental consolidation in the right upper lobe (Fig. 1). A chest computed tomography scan displayed a calcified hilar lymph node which completely obstructed the lumen of the anterior segmental bronchus of the right upper lobe, with resultant distal segmental obstructive pneumonia, which was suggestive of broncholithiasis (Fig. 1). Chest computed tomography revealed no central bronchiectasis in the contralateral left lung.
The patient then underwent a bronchoscopy, during which mucous plugging was detected, which completely obstructed the anterior segment of the right upper lobe. Microscopically, this mucous plug was determined to contain abundant eosinophils, Charcot-Leyden crystals, and fungal hyphae (Fig. 2). At this time, all cultures were negative, including a fungal culture for Aspergillus fumigatus.
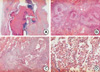
Due to this patient's unusual presentation of ABPA, as well as the associated broncholithiasis and distal obstructive pneumonia, we opted to perform a right upper lobectomy. A histopathological review of the surgically excised lung tissue specimens revealed large bronchial dilations, in which the bronchial lumen was filled with mucoid material and an inflammatory infiltrate. A distinctive exudative bronchiolitis was present distal to regions of bronchocentric granulomatosis, and this lesion was characterized by bronchiolar lumens filled with necrotic neutrophils and eosinophils, in a basophilic mucinous exudate. Foci of eosinophilic pneumonia were also observed, and noninvasive fungal hyphae were identified (Fig. 3).
Her postoperative course indicated good clinical improvement of her symptoms without steroid or antifungal treatment. Peripheral eosinophil counts were normalized and the total serum IgE after 4 months of surgery dropped to 70 U/mL.
The case of ABPA reported here was exceptional for a couple of reasons. Firstly, this was a case of ABPA in a patient exhibiting no evidence of asthma and, secondly, our radiographic findings revealed a mass-like consolidation coupled with broncholithiasis.
Apart from the bronchial asthma, our patient fulfilled seven of the eight major diagnostic criteria for ABPA (3, 6). Pulmonary infiltrates, when coupled with eosinophilia in an asthmatic subject, have raised an initial suspicion of some kind of eosinophilic lung disease such as ABPA, chronic eosinophilic pneumonia, or Churg-Strauss syndrome (7). However, ABPA is only very rarely observed in the absence of asthma. This trend is so pronounced that bronchial asthma has classically been considered an essential diagnostic criterion for ABPA, and has also been believed to play a crucial role in the development of the disease (3, 6, 8, 9). However, some cases of ABPA have been reported in non-asthmatic patients, albeit extremely infrequently, with less than 20 cases ever having been described in the literature (10-14).
Our case is rather interesting, as the patient initially presented with localized obstructive pneumonia coupled with broncholithiasis. Broncholithiasis is defined as the presence of calcified or ossified material within the lumen of the bronchus (15). A broncholith is normally formed via the extrusion of a calcified adjacent lymph node into the bronchial lumen, and is usually associated with long-standing foci of necrotizing granulomatous lymphadenitis, which are also often observed in cases of tuberculosis (15). Broncholithiasis is sometimes associated with secondary infection after the obstruction of the distal portion of the lung. Interestingly, to the best of our knowledge, no previous studies have reported a case of ABPA that was accompanied by broncholithiasis.
ABPA results from a chronic inflammatory reaction in the airway, usually a reaction to the fungal Aspergillus species. The success of this fungal strain with regard to the colonization of the lungs may be related to its size, as well as its ability to evade local clearance mechanisms. Therefore, it is possible that the localized form of ABPA in our non-asthmatic patient resulted from the impairment of local clearance mechanisms due to the broncholithiasis, combined with repeated inhalation of Aspergillus spores.
Mucoid impaction is a pulmonary disease which is characterized by the obstruction of the larger bronchi by mucous plugs. The microscopic finding of mucoid impaction of bronchi in conjunction with the presence of noninvasive fungal hyphae is extremely suggestive of ABPA (16, 17). Some researchers have suggested that mucoid impaction of bronchi represents a localized form of ABPA (18). The pathologic features of the bronchoscopic biopsy specimens and surgically excised lung tissue specimens in our case revealed a combination of mucoid impaction of bronchus, bronchocentric granulomatosis, eosinophilic pneumonia, and the presence of noninvasive fungal hyphae. These findings were consistent with previous reports on the pathologic features of ABPA (16, 19).
Greenberger and Patterson (20) and Greenberger (21) considered that patients can be classified into five stages, namely, acute stage, remission, exacerbation, corticosteroid-dependent asthma, and fibrotic end stage. These facts suggest that our patient might have been at a stage preceding bronchial asthma and it is probable that bronchial asthma may develop later.
In this report, we described a rare case of ABPA coupled with broncholithiasis. ABPA should be included in the differential diagnosis of localized pneumonic consolidations coupled with broncholithiasis, even in non-asthmatic patients.
Figures and Tables
Fig. 1
(A, B) Posteroanterior and lateral chest radiographs revealed a mass-like segmental consolidation (arrows) in the anterior segment of the right upper lobe. (C) A contrast-enhanced computed tomography (CT) scan (5-mm-collimation) displayed a 12-mm calcified hilar lymph node (arrow), which completely obstructs the lumen of the anterior segmental bronchus of the right upper lobe, with a resultant distal segmental obstructive pneumonia which was suggestive of broncholithiasis. (D) Non-enhanced, high resolution (1-mm-collimation) CT scan obtained at a lower level to C revealed a slightly high-attenuation branching structure (arrows) within the segmental consolidation, which is suggestive of mucoid impaction within the ectatic subsegmental bronchi. Note the small calcified nodule (arrowhead) in the right upper lobe, which suggests a calcified granuloma resulting from an old tuberculous infection, together with several calcified lymph nodes in the right hilar and paratracheal regions (not shown here).
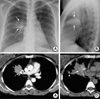
Fig. 2
Bronchoscopic biopsy specimen. Photomicrography of the impacted mucoid material revealed parallel rows of necrotic eosinophils and cellular debris, within a mucinous background (H&E, ×100). Inset: Branching fungal hyphae within impacted mucus, suggestive of Aspergillus species (GMS, ×400)
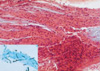
Fig. 3
Histologic sections of resected lung specimen. (A) Low-magnification photomicrography showed a dilated bronchus with mucus impaction (H&E, ×1). (B) Peribronchial granulomas with central necrosis (H&E, ×40). (C) Bronchocentric inflammation and granuloma (H&E, ×100). (D) Eosinophilic pneumonia (H&E, ×100).
References
1. Greenberger PA, Smith LJ, Hsu CC, Roberts M, Liotta JL. Analysis of bronchoalveolar lavage in allergic bronchopulmonary aspergillosis: divergent responses of antigen-specific antibodies and total IgE. J Allergy Clin Immunol. 1988. 82:164–170.

2. Laufer P, Fink JN, Bruns WT, Unger GF, Kalbfleisch JH, Greenberger PA, Patterson R. Allergic bronchopulmonary aspergillosis in cystic fibrosis. J Allergy Clin Immunol. 1984. 73:44–48.

3. Vlahakis NE, Aksamit TR. Diagnosis and treatment of allergic bronchopulmonary aspergillosis. Mayo Clin Proc. 2001. 76:930–938.

4. Neeld DA, Goodman LR, Gurney JW, Greenberger PA, Fink JN. Computerized tomography in the evaluation of allergic bronchopulmonary aspergillosis. Am Rev Respir Dis. 1990. 142:1200–1205.

5. Ward S, Heyneman L, Lee MJ, Leung AN, Hansell DM, Muller NL. Accuracy of CT in the diagnosis of allergic bronchopulmonary aspergillosis in asthmatic patients. AJR Am J Roentgenol. 1999. 173:937–942.

6. Rosenberg M, Patterson R, Mintzer R, Cooper BJ, Roberts M, Harris KE. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med. 1977. 86:405–414.

8. Choi DC. Controversial points in the diagnosis of allergic bronchopulmonary aspergillosis. Korean J Allergy. 1997. 17:506–509.
9. Park SK. Diagnosis and treatment of allergic bronchopulmonary aspergillosis. Tuberc Respir Dis. 1998. 45:687–696.

10. Glancy JJ, Elder JL, McAleer R. Allergic bronchopulmonary fungal disease without clinical asthma. Thorax. 1981. 36:345–349.

11. Berkin KE, Vernon DR, Kerr JW. Lung collapse caused by allergic bronchopulmonary aspergillosis in non-asthmatic patients. Br Med J (Clin Res Ed). 1982. 285:552–553.

12. Hoshino H, Tagaki S, Kon H, Shibusa T, Takabatake H, Fujita A, Sekine K, Abe S. Allergic bronchopulmonary aspergillosis due to Aspergillus niger without bronchial asthma. Respiration. 1999. 66:369–372.
13. Sanchez-Alarcos JM, Martinez-Cruz R, Ortega L, Calle M, Rodriguez-Hermosa JL, Alvarez-Sala JL. ABPA mimicking bronchogenic cancer. Allergy. 2001. 56:80–81.

14. Shah A, Maurya V, Panjabi C, Khanna P. Allergic bronchopulmonary aspergillosis without clinical asthma caused by Aspergillus niger. Allergy. 2004. 59:236–237.

15. Seo JB, Song KS, Lee JS, Goo JM, Kim HY, Song JW, Lee IS, Lim TH. Broncholithiasis: review of the causes with radiologic-pathologic correlation. Radiographics. 2002. 22:S199–S213.

16. Bosken CH, Myers JL, Greenberger PA, Katzenstein AL. Pathologic features of allergic bronchopulmonary aspergillosis. Am J Surg Pathol. 1988. 12:216–222.

17. Aubry MC, Fraser R. The role of bronchial biopsy and washing in the diagnosis of allergic bronchopulmonary aspergillosis. Mod Pathol. 1998. 11:607–611.
18. Scheer BG, Hutcheson PS, Lagos J, Wood J, Slavin RG. Mucoid impaction: a localized form of allergic bronchopulmonary aspergillosis. Allergy Asthma Proc. 2004. 25:229–232.
19. Jelihovsky T. The structure of bronchial plugs in mucoid impaction, bronchocentric granulomatosis and asthma. Histopathology. 1983. 7:153–167.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download