Abstract
Minoxidil induces hair growth in male pattern baldness and prolongs the anagen phase. All-trans retinoic acid (ATRA) has been reported to act synergistically with minoxidil in vivo: they can enhance more dense hair regrowth than either compound alone. We evaluated the effect of minoxidil combined with ATRA on hair growth in vitro. The effect of co-treatment of minoxidil and ATRA on hair growth was studied in hair follicle organ culture. In cultured human dermal papilla cells (DPCs) and normal human epidermal keratinocytes, the expressions of Erk, Akt, Bcl-2, Bax, P53 and P21 were evaluated by immunoblot analysis. Minoxidil plus ATRA additively promoted hair growth in vitro, compared with minoxidil alone. In addition, minoxidil plus ATRA elevated phosphorylated Erk, phosphorylated Akt and the ratio of Bcl-2/Bax, but decreased the expressions of P53 and P21 more effectively than by minoxidil alone. Our results suggest that minoxidil plus ATRA would additively enhance hair growth by mediating dual functions: 1) the prolongation of cell survival by activating the Erk and Akt signaling pathways, and 2) the prevention of apoptosis of DPCs and epithelial cells by increasing the ratio of Bcl-2/Bax and downregulating the expressions of P53 and P21.
Minoxidil, a pyrimidine derivative (2,4-diamino-6-piperidino-pyrimidine-3-oxide), is an adenosine triphosphate (ATP)-sensitive potassium channel (KATP channel) opener, and is reported to exert growth-promoting effects on follicular epithelial cells and to stimulate the rapid anagen induction of hair cycles (1, 2). Although the mechanism of minoxidil promoting hair growth is still speculative, minoxidil was reported to mediate its effect through the growth factors-related ways, to increase blood circulation around hair follicles and in addition, markedly to elevate 17β-hydroxysteroid dehydrogenase activity that accelerates the conversion of testosterone to weaker androgen (extensively reviewed in [3]). Recently, it was also reported that the expression of genes encoding potassium channels and related genes was upregulated in hair follicle bulge stem cells (4).
Vitamin A and its active derivatives such as retinoic acid have been reported to play an important role in the growth, differentiation and maintenance of hair follicles (5, 6). Topical application of all-trans retinoic acid (ATRA) is known to control hair growth cycle (7). ATRA has been suggested to stimulate the growth of sub-optimal hairs and act synergistically with minoxidil, producing more dense hairs than either compound alone (8). ATRA was also reported to enhance the percutaneous absorption of minoxidil by increasing the stratum corneum permeability (9).
Recently, we have shown that minoxidil prolongs anagen stage resulting in promoting hair growth by engaging two mechanisms: the activation of Erk and Akt that enhances the survival of cultured human dermal papilla cells (DPCs) and the increase of the ratio of Bcl-2/Bax that protects cells against cell death (10). The aim of this study was to evaluate the possible interaction of minoxidil and ATRA on hair growth in vitro, and to investigate the effect of minoxidil in combination with ATRA on normal human epidermal keratinocytes (NHK) as an epithelial counterpart in addition to the suggested system in cultured dermal papilla cells (DPCs).
Minoxidil and ATRA were purchased from Sigma (St Louis, MO, U.S.A.). Minoxidil was dissolved in 0.12 mM HCl and 1.0 mM minoxidil stock solution was stored at -20℃. ATRA was dissolved in dimethylsulfoxide (DMSO). 0.1 mM ATRA stock solution was shielded from the light with aluminum foil until required.
Anti-phosphorylated Erk-1/2 (Thr202/Tyr 204) antibody, anti-total Erk-1/2 antibody, anti-phosphorylated Akt (Ser473) antibody, and anti-total Akt antibody were purchased from Cell Signaling Technology, Inc. (Beverly, MA, U.S.A.). Anti-Bcl-2 and anti-Bax antibodies were obtained from Dako (Glostrup, Denmark). Anti-P53 (DO7) antibody was obtained from Novocastra (Newcastle, U.K.). Anti-P21 antibody was obtained from Oncogene (San Diego, CA, U.S.A.). Anti-β-actin antibody was obtained from Santa Cruz Biotech Inc. (Santa Cruz, CA, U.S.A.).
Tissue samples of the occipital scalp region were obtained by excisional biopsy. Ten healthy male volunteers, aged between 20 and 35 yr, were recruited. The subjects did not have a current or prior disease and were not under medication for at least 1 month. The Institutional Review Board at the Seoul National University Hospital approved all procedures used in this study. Written informed consents were obtained from all volunteers. Tissue samples containing more than 100 hair follicles were cautiously dissected into single hair follicles. We used hair follicles morphologically in the anagen stage only determined as described previously (11).
Human scalp hair follicles were isolated and cultured in vitro as described previously (12). Briefly, dissected hair follicle was cut into small pieces approximately 2.5 mm in length from the bottom of dermal papilla and cultured in Williams E medium (Gibco BRL, Gaithersburg, MD) with 10 ng/mL hydrocortisone, 10 µg/mL insulin, 2 mM L-glutamine, and 100 U/mL penicillin at 37℃ in 5% CO2 atmosphere. Minoxidil alone or in combination with ATRA was added to the culture medium. Minoxidil was used at 1 µM. ATRA was used at the final concentration ranged from 1 nM to 10 nM.
A total of 180 anagen hair follicles from 3 different volunteers (60 follicles per subject) were cultured in triplicates under 4 different growth conditions. The data are presented as the means±SEM. In all experiments, tissue culture medium containing minoxidil alone or minoxidil plus ATRA was changed every other day. After culturing for 12 days, the hair growth was measured directly using an Olympus stereomicroscope with an eyepiece containing a graticule.
Human DPCs were cultured as described previously (13). Briefly, DPCs were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco BRL, Gaithersburg, MD, U.S.A.) containing 2 mM L-glutamine, 1× antibiotic antimycotic solution (1,000 units/mL of penicillin G sodium and 2.5 µg/mL of amphotericin B) and 10% fetal bovine serum (Hyclone, Logan, UT, U.S.A.) at 37℃, in a 5% CO2 incubator. The fourth-passage DPCs were used.
As follicular and nonfollicular epidermal keratinocytes are known to respond similarly to minoxidil treatment (14), we used NHK of five human adult foreskins obtained from circumcisions. NHK were isolated as previously described (15) and cultured in keratinocyte growth medium (Clonetics, San Diego, CA, U.S.A.) composed of MCDB 153 medium supplemented with epidermal growth factor (10 ng/mL), bovine pituitary extract (70 µg/mL), hydrocortisone (0.5 µg/mL), insulin (5 µg/mL), penicillin (100 µg/mL) and fungizone (0.25 µg/mL). The NHK were cultured until 80% confluent in tissue culture flasks (Becton Dickinson, Lincoln Park, NJ, U.S.A.) at 37℃ and 5% CO2 and stored in liquid nitrogen until use. At the time of the experiments, the cells were thawed and third passage cells were used.
As a parameter to check for cytotoxic effect of minoxidil plus ATRA, cell viability was measured using the MTT assay as previously described (16). DPCs and NHK of 1.5×104 cells/well were seeded into 96-well plates, cultured for 24 hr in serum-free DMEM, and treated with the vehicle (0.12 mM HCl/DMSO diluted 1:1,000 in DMEM) as control, and the various combined concentrations of ATRA (0.01-1 nM) and minoxidil (0.01-1.0 µM) for 5 days. 20 µL MTT solution (5 mg/mL) was added to a well, incubated for 4 hr at 37℃ in the dark, removed the supernatants, added 200 µL DMSO to dissolve formazan products, incubated for 30 min at room temperature, and the absorbance was measured at 570 nm using an ELISA reader. The results were expressed as the percentage of control cells in six same culture conditions.
For the treatment with minoxidil alone or minoxidil plus ATRA, confluent DPCs and NHK were incubated for 24 hr in serum-free DMEM and treated with the vehicle (0.12 mM HCl and/or DMSO diluted at 1:1,000 in DMEM in half), minoxidil alone (0.1 µM), or 0.1 µM minoxidil plus 0.1 nM ATRA for 1 hr for the evaluation of Erk and Akt, and for 24 hr for the evaluation of Bcl-2/Bax and P53/P21.
Western blot analysis was performed as follows. Briefly, proteins were extracted using the buffer containing 50 mM Tris-HCl (pH 7.4), 2 mM EDTA, 100 µg/mL leupeptin, 20 µg/mL aprotinin, and 100 mM NaCl. The supernatant was collected and kept at -70℃ until used. Fifty microgram of protein was loaded to a lane of 10% or 12% SDS-polyacrylamide gel, separated by electrophoresis, and blotted onto nitrocellulose membrane. The blotted membrane was incubated with primary antibody (anti-total Erk polyclonal antibody, 1:500; anti-phosphorylated Erk polyclonal antibody, 1:500; anti-total Akt polyclonal antibody, 1:500; anti-phosphorylated Akt polyclonal antibody, 1:500; anti-Bcl-2 monoclonal antibody, 1:1,000; anti-Bax monoclonal antibody, 1:1,000; anti-P53 monoclonal antibody, 1:1,000; anti-P21 monoclonal antibody, 1:1,000; anti-β-actin monoclonal antibody, 1:1,000) at 4℃ overnight. The membrane was incubated with the anti-mouse IgG-HRP conjugate (1:2,000) or the anti-rabbit IgG-HRP conjugate (1:2,000) for 1 hr at room temperature. The antibody-antigen complex was detected using the ECL system (Amersham Pharmacia Biotech; Little Chalfont, U.K.) and analyzed using a Bio-Rad GS-700 imaging densitometer (Hercules, CA, U.S.A.).
Compared with minoxidil alone, the growth-promoting activity of the minoxidil plus ATRA was examined in in vitro human hair follicle culture model. Minoxidil at 1 µM enhanced hair growth significantly compared with vehicle-treated control (p<0.05) (Fig. 1). The minoxidil plus ATRA (1 nM) more increased hair growth by 4.2±0.7 mm compared with mioxidil alone (p<0.05). Combination of the higher concentration of ATRA (10 nM) was less effective than the mixture of minoxidil plus 1 nM ATRA, although the enhanced hair growth by mixed preparation with 10 nM ATRA was at least not less than the minoxidil alone.
Minoxidil (0.01-1 µM) plus ATRA (0.01-1 nM) enhanced the proliferations of cultured DPCs and NHK. The enhancement was the greatest at minoxidil 0.1 µM plus ATRA 0.1 nM by 189.7±42.1% (mean±SD) in DPCs and by 219.5±57.8% in NHK.
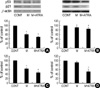
Treated with minoxidil, the phosphorylated Erk (p-Erk) level in DPCs was significantly increased to 214±21%. The minoxidil plus ATRA more significantly elevated the p-Erk level to 298±50% (p<0.05) (Fig. 2A). The NHK from five different donors was tested with minoxidil alone or minoxidil plus ATRA and in the experiment, it has to be mentioned that NHK from 2 donors did not respond to the treatment. The expression of p-Erk in other three samples was slightly elevated to 106±4% by treatment of minoxidil. Minoxidil plus ATRA increased the expression of p-Erk to 207±51% (Fig. 2B).
The total Erk level was barely changed in response to minoxidil alone or minoxidil plus ATRA in both cells. The values shown are the means±SEM of percentage increases versus the control from three different batches of DPCs or NHK.
Minoxidil enhanced the level of phosphorylated Akt (p-Akt) to 126±8% in DPCs and the minoxidil plus ATRA more increased the phosphorylation of Akt to 315±8% (p<0.05) (Fig. 3A).
The expression of p-Akt in the NHK from the three donors increased in a similar manner with DPCs by minoxidil alone and minoxidil plus ATRA to 111±10% and 284±56%, respectively (Fig. 3B). The expression of p-Akt was not elevated in the same two cells that were irresponsive to the treatment in p-Erk. Minoxidil alone and minoxidil plus ATRA had no effect on the expression of total Akt in both cells.
To evaluate the possible association in changes on Bcl-2 family proteins, we investigated the effects of minoxidil alone or minoxidil plus ATRA on the expressions of Bcl-2 and Bax protein. Treated with minoxidil, the expression of Bcl-2 protein was increased in DPCs to 128±18%. The increase, however, was not statistically significant. Minoxidil plus ATRA elevated the Bcl-2 expression to 200±73% with statistical significance (p<0.05) (Fig. 4A). For NHK, the expression of Bcl-2 protein was increased similarly; elevated Bcl-2 expression to 206±38% with minoxidil alone, and with minoxidil plus ATRA, to 325±68% (Fig. 4B).
In contrast, minoxidil decreased the Bax protein expression significantly in DPCs, to 75±3%. Minoxidil plus ATRA more significantly downregulated the expression of Bax to 45±10% (p<0.05) (Fig. 4C). Minoxidil alone or minoxidil plus ATRA also decreased the Bax protein expression significantly in NHK to 83±7% and to 47±9%, respectively (p<0.05) (Fig. 4D).
P53 plays a pivotal role in the regulation of cell growth and programmed cell death. P21 is a specific downstream target of P53 that mediates the cell cycle arrest (17). Treated in DPCs, minoxidil alone and minoxidil plus ATRA down-regulated the expression of P53 to 59±2% and to 47.5±10%, respectively (p<0.05) (Fig. 5A). For NHK, expression patterns of P53 were also decreased significantly by minoxidil plus ATRA (p<0.05) (Fig. 5B).
All-trans retinoic acid, alone or in combination with minoxidil, has been reported to shorten the second telogen phase and lengthen the second anagen phase in the C3H mouse model (7). In this study, we demonstrated that the minoxidil plus ATRA additively enhances hair growth in in vitro human hair follicles, compared with minoxidil alone.
Previously, minoxidil was reported to have the concentration-dependent biphasic effect on proliferation and differentiation; growth stimulation at low doses or anti-proliferative, pro-differentiative and partially cytotoxic effects at high doses (14). At 10 nM of ATRA co-treated with minoxidil, less elongation than at 1 nM suggested that ATRA might increase the tissue concentration of minoxidil in hair follicles in vitro.
Although ATRA is converted to other active metabolites in the dermis, the doses used in organ culture would be compatible to the dermal concentration in vivo, because the concentration of commercially available topical ATRA is 0.01-0.1% and in human cadaver skin, 0.11-0.44% of ATRA is deposited in the dermis by topical application (18).
The cellular and molecular basis of ATRA mediating cell proliferation and the formation of extracellular matrix has not been delineated. ATRA at 1 nM was reported to extend the life span of human oral and epidermal keratinocytes by decreasing the expression of senescence-associated genes (p53, p21, p16) and maintaining telomerase activity, but at higher concentrations the replicative senescence was enhanced in a dose-dependent manner (19).
We demonstrated here that minoxidil plus ATRA additively increased the phosphorylation of Erk in both DPCs and NHK, compared with minoxidil alone. The role of the Erk signaling pathway in cell growth has been well established (20). The diverse involvement of retinoic acid has been demonstrated in the regulation of developmental processes and the modulation of differentiation in various cellular models by the MAPK pathways (21, 22). The MAPK pathway is also involved in maintaining cell survival by modulating apoptotic molecules including Bcl-2 family (23). The PI3 kinase/ Akt cascade plays a crucial role in cell survival and the prevention of apoptosis (24, 25). The crosstalk between Erk and PI3K/Akt pathway has been demonstrated to prolong cell survival (26). Our results thus suggest that the activation of Akt, an anti-apoptotic molecule, by minoxidil plus ATRA may prolong survival of DPCs and epithelial counterpart.
The Bcl-2 family proteins are structurally related molecules that play an essential role in the regulation of apoptosis (27). Bcl-2 family consists of anti-apoptotic proteins such as Bcl-2 as well as pro-apoptotic proteins such as Bad and Bax. Erk and PI3K/Akt pathways synergistically induce cell survival by Bcl-2 cascade (28). Throughout the hair cycle, dermal papilla (DP) is the only region expressing consistently Bcl-2 proteins and the Bax proteins are not detected during hair cycles (29, 30). The DP thus is considered to be resistant apoptosis. On the other hand, the downregulation of Bcl-2 expression has been shown to induce apoptosis of DPCs in tissue culture (31). The relative amount of Bcl-2 over Bax is known to determine the fate of living cells (32). Retinoid treatment has been reported to increase the ratio of mRNAs encoding bcl-2 and bax significantly (33). Our data suggest that minoxidil plus ATRA additively promote cell survival by the modulation of the ratio of Bcl-2 and Bax in DPCs and NHK.
P53 is a tumor suppressor gene that plays a pivotal role in the regulation of cell growth and cell death. The downstream transcriptional targets of P53 are the cyclin-dependent kinase inhibitor P21WAF1 that mediates G1 arrest and Bax that is involved in apoptosis (17). In addition, bcl-2 gene was demonstrated to act as a transcriptional target for wild-type P53 that downregulates the endogenous Bcl-2 expression and upregulates the Bax expression (34). Moreover, in addition to transcriptional activation, P53-dependent apoptosis may be induced through a transactivation-independent means, such as by the induction of oxidative stress, the inhibition of RNA, or the inhibition of protein synthesis (35). In this study, minoxidil plus ATRA worked together to suppress the expression of P53 and its downstream target P21, although the exact mechanism has to be elucidated in future experiments.
In summary, our data suggest that the enhanced hair growth by minoxidil plus ATRA in our short-term organ culture may be explained through prolonged survival of epithelial cells and DPCs, which mediate signals for follicular epithelium. Minoxidil plus ATRA more increased the phosphorylation of Erk and Akt early 1 hr after the treatment than minoxidil alone. The change of Bcl-2/Bax ratio, P53 and P21 were also detected later 24 hr after the treatments. Minoxidil plus ATRA could work together to prolong the survival of cultured DPCs and epithelial cells and to protect them from apoptosis by dual mechanisms with different kinetics: 1) the activation of Erk- and Akt-dependent pathways and 2) the increase of the ratio of Bcl-2/Bax and the suppression of the expression of P53 and its downstream target P21.
Figures and Tables
Fig. 1
The effect of minoxidil plus ATRA on hair growth in vitro. Hair follicle organ culture was performed in triplicates with hair follicles obtained from the occipital scalp of three volunteers. Hair growth is enhanced by minoxidil at 1 µM. Minoxidil plus ATRA promotes hair growth more significantly, especially at a concentration of 1 µM minoxidil and 1 nM ATRA. Moreover, the increase in hair growth was significant compared with minoxidil alone. Values were obtained after cumulative growth of 15 hair follicles per each growth condition for 12 days and shown as the means±SEM. *, p<0.05; †, p<0.01.
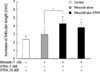
Fig. 2
The effect of minoxidil and ATRA on the p-Erk and on total Erk in (A) DPCs and in (B) NHK. The level of p-Erk increased significantly after treatment of minoxidil, and minoxidil plus ATRA more enhanced the expression of p-Erk. The representative bands of triplicate experiments are shown. The values shown are the means±SEM of % elevation compared with the vehicle-treated control from three different DPCs and NHK cultures. *, p<0.05, compared with the vehicle-treated control. p-Erk, phosphorylated Erk; T-Erk, total Erk; M, minoxidil.
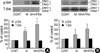
Fig. 3
The increase of p-Akt expression by minoxidil and ATRA. (A) In DPCs, minoxidil increases p-Akt, moreover, minoxidil plus ATRA more significantly elevates the p-Akt level. (B) In NHK, minoxidil alone and minoxidil plus ATRA increase p-Akt expression, however, they were statistically insignificant. The blotted bands are the representative of triplicate experiments. The values are shown as the means±SEM of % elevation compared with controls. *, p<0.05, compared with controls treated with the vehicle. p-Akt, phosphorylated Akt; T-Akt, total Akt; M, minoxidil.
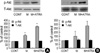
Fig. 4
The effect of minoxidil and ATRA on the expression of Bcl-2 and Bax protein in DPCs and NHK. Compared with vehicle-treated control, minoxidil plus ATRA significantly increases the expression of Bcl-2 in DPCs (A) and in NHK (B). (C) Minoxidil significantly decreases the expression of Bax. Minoxidil plus ATRA suppresses the expression of Bax more efficiently in DPCs. (D) Expression of Bax in NHK is also significantly decreased by minoxidil plus ATRA. The bands are the representatives of triplicate experiments. The values are shown as the means±SEM of % elevation in comparison with the controls. *, p<0.05 compared with vehicle-treated control. M, minoxidil.
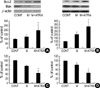
Fig. 5
The effects of minoxidil and ATRA on the expressions of P53 and P21 protein in DPCs and NHK. (A) Minoxidil alone and minoxidil plus ATRA significantly suppress the expression of P53 protein in DPCs. (B) In NHK, minoxidil plus ATRA decreases P53 expression significantly. (C) In DPCs, Minoxidil significantly decreases the expression of P21. Minoxidil plus ATRA reduces the expression of P21 more significantly. (D) P21 expression is significantly downregulated with minoxidil plus ATRA. The bands are the representative of triplicate experiments. *, p<0.05 compared with the vehicle-treated control. The data are shown as the means±SEM of % elevation compared with controls from three different DPC and NHK cultures. M, minoxidil.
ACKNOWLEDGEMENT
We thank for excellent statistical assistance to Min Jeong Lee in Clinical Research Institute, Seoul National University Hospital.
References
1. Tanigaki-Obana N, Ito M. Effects of cepharanthine and minoxidil on proliferation, differentiation and keratinization of cultured cells from the murine hair apparatus. Arch Dermatol Res. 1992. 284:290–296.

2. Lee WS, Ahn HJ, Kim YH. The effect of coapplication of capsaicin and minoxidil on the murine hair growth. Korean J Dermatol. 2003. 41:451–460.
3. Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004. 150:186–194.

4. Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004. 22:411–417.

6. Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996. 10:1002–1013.

7. Bazzano G, Terezakis N, Attia H, Bazzano A, Dover R, Fenton D, Mandir N, Celleno L, Tamburro M, Jaconi S. Effect of retinoids on follicular cells. J Invest Dermatol. 1993. 101:138S–142S.

8. Bazzano GS, Terezakis N, Galen W. Topical tretinoin for hair growth promotion. J Am Acad Dermatol. 1986. 15:880–883. 890–893.

9. Ferry JJ, Forbes KK, VanderLugt JT, Szpunar GJ. Influence of tretinoin on the percutaneous absorption of minoxidil from an aqueous topical solution. Clin Pharmacol Ther. 1990. 47:439–446.

10. Han JH, Kwon OS, Chung JH, Cho KH, Eun HC, Kim KH. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J Dermatol Sci. 2004. 34:91–98.

11. Messenger AG. The culture of dermal papilla cells from human hair follicles. Br J Dermatol. 1984. 110:685–689.

13. Randall VA, Thornton MJ, Redfern CP. Dermal papilla cells from human hair follicles express mRNA for retinoic acid receptors in culture. Ann N Y Acad Sci. 1991. 642:457–458.

14. Boyera N, Galey I, Bernard BA. Biphasic effects of minoxidil on the proliferation and differentiation of normal human keratinocytes. Skin Pharmacol. 1997. 10:206–220.

15. Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983. 81:33s–40s.

16. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. 65:55–63.

17. Kim R, Tanabe K, Emi M, Uchida Y, Inoue H, Toge T. Inducing cancer cell death by targeting transcription factors. Anticancer Drugs. 2003. 14:3–11.

18. Skov MJ, Quigley JW, Bucks DA. Topical delivery system for tretinoin: research and clinical implications. J Pharm Sci. 1997. 86:1138–1143.

19. You YO, Lee G, Min BM. Retinoic acid extends the in vitro life span of normal human oral keratinocytes by decreasing p16 (INK4A) expression and maintaining telomerase activity. Biochem Biophys Res Commun. 2000. 268:268–274.
20. Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997. 9:180–186.

21. Bost F, Caron L, Marchetti I, Dani C, Le Marchand-Brustel Y, Binetruy B. Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage. Biochem J. 2002. 361:621–627.

22. Chung JH, Kang S, Varani J, Lin J, Fisher GJ, Voorhees JJ. Decreased extracellular-signal-regulated kinase and increased stress-activated MAP kinase activities in aged human skin in vivo. J Invest Dermatol. 2000. 115:177–182.

23. Chang F, Steelman LS, Shelton JG, Lee JT, Navolanic PM, Blalock WL, Franklin R, McCubrey JA. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway [Review]. Int J Oncol. 2003. 22:469–480.
24. Ahmad S, Singh N, Glazer RI. Role of AKT1 in 17beta-estradiol- and insulin-like growth factor I (IGF-I)-dependent proliferation and prevention of apoptosis in MCF-7 breast carcinoma cells. Biochem Pharmacol. 1999. 58:425–430.
25. Tang Y, Zhou H, Chen A, Pittman RN, Field J. The Akt proto-oncogene links Ras to Pak and cell survival signals. J Biol Chem. 2000. 275:9106–9109.

26. Chang F, Lee JT, Navolanic PM, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003. 17:590–603.

27. Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003. 22:8590–8607.

28. McCubrey JA, Steelman LS, Blalock WL, Lee JT, Moye PW, Chang F, Pearce M, Shelton JG, White MK, Franklin RA, Pohnert SC. Synergistic effects of pi3k/akt on abrogation of cytokine-dependency induced by oncogenic raf. Adv Enzyme Regul. 2001. 41:289–323.

29. Stenn KS, Lawrence L, Veis D, Korsmeyer S, Seiberg M. Expression of the bcl-2 protooncogene in the cycling adult mouse hair follicle. J Invest Dermatol. 1994. 103:107–111.

30. Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R. Analysis of apoptosis during hair follicle regression (catagen). Am J Pathol. 1997. 151:1601–1617.
31. Ferraris C, Cooklis M, Polakowska RR, Haake AR. Induction of apoptosis through the PKC pathway in cultured dermal papilla fibroblasts. Exp Cell Res. 1997. 234:37–46.

32. Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998. 281:1322–1326.

33. Chung JJ, Cho S, Kwon YK, Kim DH, Kim K. Activation of retinoic acid receptor gamma induces proliferation of immortalized hippocampal progenitor cells. Brain Res Mol Brain Res. 2000. 83:52–62.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download