Abstract
Cadmium is known to exert toxic effects on multiple organs, including the testes. To determine if α-Tocopherol, an antioxidant, could protect testicular tissues and spermatogenesis from the toxic effects of cadmium, six-week old male Sprague-Dawley rats were randomized to receive cadmium at doses of 0 (control), 1, 2, 4 or 8 mg/kg by the intraperitoneal route (Group A) or α-tocopherol for 5 days before being challenged with cadmium (Group B) in an identical dose-dependent manner. When both groups received cadmium at 1 mg/kg, there were no changes in testicular histology relative to controls. When Group A received cadmium at 2 mg/kg, undifferentiated spermatids and dead Sertoli cells increased in the seminiferous tubules while interstitial cells decreased and inflammatory cells increased in the interstitial tissues. On flow cytometric analysis, the numbers of elongated spermatids (M1) and round spermatids (M2) decreased while 2c stage cells (M3, diploid) increased. In contrast, when Group B received cadmium at 2 mg/kg, the histological insults were reduced and the distribution of the germ cell population remained comparable to controls. However, α-tocopherol had no protective effects with higher cadmium doses of 4 and 8 mg/kg. These findings indicate that α-tocopherol treatment can protect testicular tissue and preserve spermatogenesis from the detrimental effects of cadmium but its effectiveness is dependent on the dose of cadmium exposed.
Cadmium is widely used as an anticorrosive in plating metals and other alloys that are valuable in industry and, as cadmium oxide, in storage batteries (1). With the wide application of cadmium-related products in industrialized nations, hazardous exposure to cadmium is increasing. Cadmium accumulates in the human body for a long time even after minimal exposure and has severe toxic effects. Cadmium has been linked to osteomalacia, hepatotoxicity, renal toxicity, neurotoxicity as well as infertility and cancer (2-9). It is known to induce lipid peroxidation (LPO) by stimulating the production of superoxide anions (10). Within the cells, cadmium accelerates LPO and suppresses antioxidants such as superoxide dismutase or glutathione peroxidase (1112). Free radicals then accumulate, leading to cell damage, aging and the development of chronic diseases.
An increase in oxidative damage to sperm membranes, proteins, and DNA is associated with alterations in signal transduction mechanisms that affect fertility (13). It has been reported that human spermatozoa are capable of spontaneous LPO and generating reactive oxygen species and that superoxide dismutase present in sperm may play a major role against LPO (14). In addition, LPO has been reported to be accelerated among defective spermatozoa exhibiting high levels of reactive oxygen species and that α-tocopherol could reverse the functional consequences of LPO (15). Alpha-tocopherol is a chain-breaking antioxidant that exists in cell membranes (16). It eliminates lipid peroxyl and alkoxyl radicals, suppresses the chain reaction of LPO and promotes the production of scavenger antioxidant enzymes (17).
The current study was undertaken to characterize the dose-response effects of cadmium on testicular weight, morphology and DNA flow cytometry in male rats, and to examine if α-tocopherol can also protect changes induced by cadmium.
Six-week old male Sprague-Dawley rats weighing between 180 and 200 g were used in the experiments. All animals were housed in our special Trace Metal Animal Facilities at a temperature range of 24±1℃, relative humidity of 45±5% and a 12 hr light-cycle. Investigations were conducted in accordance with the Guide for Care and Use of Laboratory Animals (1996, National Academy of Science).
These male rats were randomly assigned to receive cadmium at doses of 0 (control), 1, 2, 4 or 8 mg/kg either alone (Group A) or after α-tocopherol treatment for 5 weeks (Group B). The numbers of rats included in Group A for final analysis were 10, 11, 8, 14 and 17 at 0, 1, 2, 4 and 8 mg/kg, respectively; those included in Group B were 8, 8, 8, 8 and 7. The corresponding cadmium dose was administered as a 0.5mL solution of cadmium chloride (Sigma C-3141, St. Louis, MO, U.S.A.) in 0.9 % normal saline by the intraperitoneal route using a 23-gauge syringe. In both groups, control rats (i.e., cadmium dose of 0 mg/kg) received normal saline only. In Group B, rats received 0.1 mL α-tocopherol (Sigma T-3251) daily for 5 days by the intraperitoneal route before administering normal saline alone or the corresponding cadmium dose one hour after the last dose of α-Tocopherol.
One week after normal saline (control) or cadmium injections, each rat was sacrificed by cervical dislocation, and the abdomen was incised to remove both testes. Each testis was then washed with normal saline to separate the surrounding fat and connective tissues. After drying the surface with filter paper, the dimensions and weight of the testis were recorded.
The left testicle of each rat was serially sectioned and fixed in Bouin solution for 48 hr. The specimen was then dehydrated through a graded series of alcohol and cleared in three changes of xylene before embedded in paraffin. Serial sections, each of 4 µm thickness, were made and stained with hematoxylin and eosin according to standard method. Histological assessment was performed under light microscopy in terms of seminiferous tubular diameter (STD: in every H&E section a minimum of 25 circular tubule were measured in two axes drawn perpendicular to each other) using an image analyzer (Image Proplus Version 3.0, Media Cybernetics, Silver Spring, MD, U.S.A.).
For flow cytometric analysis, testicular samples were prepared and stained with minor modification according to the method described by Zante et al. (18). The right testis of each rat was collected in Dulbecco's phosphate-buffered saline (PBS), minced and filtered through a 30 µm nylon filter to form a cellular suspension. After centrifugation at 500 g for 5 min, samples were fixed with 70% ethyl alcohol and kept at -20℃. Prior to flow cytometric analysis, the fixed cells were centrifuged at 500 g for 5 min. The cells were then resuspended (1×106 cells/mL) in PBS containing propidium iodide (Sigma) and 100 µL of RNase (Sigma) and kept in the dark until DNA analysis. DNA analysis was performed on the FACScalibur (Becton Dickinson, San Jose, CA, U.S.A.) equipped with a 488 nm argon laser. During analysis, the flow rate was controlled at 500 cells/sec approximately, and for each sample, at least 10,000 events were recorded. The data were processed on a computer equipped with CELLQuest software (Version 3.1; Becton Dickinson). A typical DNA histogram of human adult testicular tissue is characterized by 4 peaks; 1) Peak I represents haploid mature spermatids with highly condensed chromatin that do not stain proportionally to their DNA content; 2) Peak II, represents haploid round spermatids with a 1c DNA content; 3) Peak III represents non-germ cells (Sertoli cells, Leydig cells and macrophages) and germ cells with a 2c DNA content (including G1-spermatogonia, primary spermatocytes at preleptotene and secondary spermatocytes); and 4) Peak IV represents mainly primary spermatocytes with a small percentage of (G2+M)-spermatogonia. It is between Peak III and IV that cells synthesizing DNA are registered. M1, M2, M3, M4, M5 and M6 cell development correspond to Peak I, Peak II, Peak III, between Peak III and Peak IV, Peak IV, and debris, respectively.
Within-group differences in testicular length, width and weight and the distribution of cells at the different stages (M1 to M6) on flow cytometry were compared by one-way analysis of variance (ANOVA). This was followed by post hoc pairwise comparison using the Bonferroni t-procedure if the overall results were significant. Statistical analysis was performed using the SPSS for Windows, Version 9.0 (Chicago, IL, U.S.A.) with statistical significance defined at a level of p<0.05.
Following treatment with cadmium alone, there was a progressive decrease in testicular length, width and weight with increasing cadmium dose beyond 1 mg/kg (p<0.01;Table 1). A similar dose-response relationship was noted in rats pretreated with α-Tocopherol. However, relative to control rats, a significant decrease in testicular weight was observed only at the higher cadmium doses of 4 and 8 mg/kg (Table 1).
At the cadmium dose of 1 mg/kg, there were no marked changes in testicular histology relative to controls irrespective of α-tocopherol treatment. Thus, normal spermatogen esis, well-preserved Sertoli cells and well-delineated tubular basement membrane were observed in both Group A and Group B. The interstitium between tubules and Leydig cells were also intact without any inflammatory cell infiltrates (Fig. 1A, B). However, at the cadmium dose of 2 mg/kg, differences in testicular histology were noted between the two groups. In rats treated with cadmium alone (Group A), there were heavy infiltrates of acute and chronic inflammatory cells in the relatively well-preserved interstitium between seminiferous tubules. Although the tubular basement membranes of seminiferous tubules were identified, tubules could exhibit focal or diffuse extensive necrosis (Fig. 1C). A few viable cells were identified in some tubules mostly composed of immature forms of germ cells and Sertoli cells. In contrast, prior α-tocopherol treatment (Group B) reduced the degree and number of necrotic seminiferous tubules (Fig. 1D). However, most of germ cells were degenerated, especially the ones involving highly differentiated germ cells (Fig. 1D inset). At the cadmium doses of 4 mg/kg, there was diffuse and marked tubular necrosis in which α-tocopherol provided no protective effects. In both groups, all the spermatogenic cells were dead. Almost all the ground substance within the interstitium disappeared and replaced by fibroblasts and inflammatory cells (Fig. 2). These features were identical in rats receiving the cadmium dose of 8 mg/kg irrespective of α-tocopherol treatment.
In flow cytometric analysis, controls (i.e., no cadmium) showed the typical 4-peak DNA dispersion observed in the normal adult testis (Fig. 3A). The cells were distributed as follows: 88.8% M1+M2 (1c: haploid spermatid), 6.1% M3 (2c: contains diploid germ cells), 0.6% M4, 3.6% M5 (including tetaploid germ cells) and 0.9% M6 (Table 2). At a cadmium dose of 1 mg/kg (Fig. 1B), the distribution was relatively unchanged (e.g., 86.1% M1+M2). However, at a cadmium dose of 2 mg/kg, M1+M2 cells decreased to 62.9% while M3 and M4 increased to 30.2% and 2.2% respectively (Fig. 3Cand Table 2). These were significantly different from control values (p<0.01).
As for Group A, the distribution in controls (i.e., no cadmium) pretreated with α-tocopherol for 5 days showed the typical 4-peak DNA dispersion observed in the normal adult testis (Fig. 3D) with 81.8% M1+M2; 10.4% M3; 1.3% M4; and 4.6% M5. Similarly, the cell distribution at a cadmium dose of 1 mg/kg (Fig. 3E) was not significantly different from that of controls. In contrast to Group A, the distribution of M1+M2, M3 and M5 at a cadmium dose of 2 mg/kg remained unchanged with prior α-tocopherol treatment (Fig. 3F and Table 2).
Spermatogenesis is a sophisticated and complex differentiation process (19). After a period of prenatal mitotic division, the germ cells present in the seminiferous cords of the testes at birth remain quiescent until immediately before puberty, when they divide by mitosis to form spermatogonia. A population of these cells then enters the pre-stage of meiosis to become spermatocytes and after meiosis, spermatids (1n chromosomes and 1c DNA). The remaining spermatogonial population in the seminiferous tubules serves as supporting cells, providing metabolic and physiologic functions. Degeneration of spermatogonia is an integral and important part of normal spermatogenesis. In the rat, available evidence suggests that cell loss in the spermatogonial stages probably exceeds 75 % (20-23). However, spermatogonial degeneration can result from exposure to toxic chemicals, heat and radiation, deficiencies of hormones or growth factors and immunodeficiency (23-26). Some of these predisposing factors such as cadmium reported here, may cause irreversible testicular damage and lead to permanent spermatogonia loss and infertility.
In our experiments, the toxic effects of cadmium on testicular histology were dose-dependent. At 1 mg/kg, cadmium had minimal effects on testicular histology but at 2 mg/kg, there were prominent necrotic changes. The critical dose in which extensive necrosis occurred was 4 mg/kg. With doses higher than 4 mg/kg, some rats lost appetite and some died. In proportion with the cadmium dose (Fig. 2), supporting cells were also destroyed, leading to irreversible loss of spermatogonia and destruction of basement membranes. Correspondingly, interstitial cell function was initially suppressed; interstitial cells then decreased in number; and finally, were irreversibly replaced by inflammatory cells. In contrast, prior α-tocopherol treatment reduced some of the early morphological changes before damage became irreversible. This was clearly demonstrated at the 2 mg/kg cadmium dose when the necrotic changes in the testes were reduced (Fig. 1D). The protective effects of α-tocopherol were also apparent in our flowcytometry experiments at the cadmium dose of 2 mg/kg. In contrast to rats receiving cadmium alone at 2 mg/kg, the cell distribution in rats receiving α-tocopherol in addition was similar to those of controls. However, when the cadmium dose increased beyond 2 mg/kg, α-tocopherol had no protective effects.
Our findings were consistent with the sequence of changes in the rat testis following cadmium administration described by Kwon et al. (27). Although there were minimal histological changes in the testis using a cadmium dose of 1 mg/kg, protein electrophoresis showed a loss of 25 and 45 kDa proteins. The authors suggested that following cadmium exposure, proteins were denatured first, particularly those most vulnerable to protease attack; protein movement inside the gel then decreased as the molecular weight increased due to cadmium binding; finally, proteins were destroyed by necrotic factors generated by cadmium.
The rat testis has 14 stages in the cycles in seminiferous epithelium and identifying the stage of development is tedious process that is likely to have intra- and inter-observer variations. To obviate these observer related variants, DNA flow cytometry has been suggested to be sensitive indicator of germ cell change in the testis (28). The spermatogonia (G2 cells) are tetraploid (4c), primary spermatocytes are diploid (2c) and spermatozoa are haploid (1c).
Immediately after radiation exposure, highly differentiated cells such as 1c cells increased while 2c, S-phase, and 4c cells decreased temporarily (29). In contrast, at cadmium doses of 1 and 2 mg/kg, M1 and M2 cells represents haploid mature spermatozoa decreased while M3 and M4 cells disclose diploid spermatocyte and tetraploid spermatogonia and were increased at a cadmium dose of 2 mg/kg. Taken together, this suggest that highly differentiated testicular cells might be more vulnerable to cadmium but more resistant to radiation. In parallel with histological changes, the extensive presence of necrotic cells at higher doses of cadmium precluded flow cytometry assessment.
Alpha-tocopherol is found in a variety of cells, including mammalian sperm. Our data are consistent with previous studies which showed a sperm-protective role of α-tocophereol. Alpha-tocopherol could suppress LPO in sperm as assessed by malondialdehyde concentration and improved sperm motility (30). In human sperm, its concentrations had been correlated with superoxide dismutase and glutathione peroxidase activities when semen samples contained 106 leukocytes/ mL (31). It could reverse the functional consequences of LPO as assessed by sperm-oocyte fusion (15). When α-tocopherol was added during sperm freezing, sperm viability was found to improve in human (32), ram (33), and bovine sperm (34). It stabilized sperm membrane integrity and suppressed LPO during sperm capacitation in the bull (35).
In conclusion, our findings confirm that cadmium can severely destroy testicular tissues and affect spermatogenesis. While α-tocopherol can protect the testis against cadmium, its effectiveness is dependent on the dose of cadmium. Whether the protective effects of α-tocopherol are also dose-dependent is unknown as we only used a fixed dose of α-tocopherol in our experiments.
Figures and Tables
Fig. 1
Histology of the rat testis and the effects of α-tocopherol : Most seminiferous tubules are well preserved in controls (A). Rats treated with cadmium 1 mg/kg (B) exhibit diffuse or focal necrosis. In rat treated with cadmium 2 mg/kg and some of the immature spermatocytes appear hypertrophic, multinucleated or degenerated (C). In contrast, most tubules are relatively well preserved in rats receiving α-tocopherol in addition to cadmium 2 mg/kg (D). Most of cells in seminiferous tubules are degenerated. Noted loss of head of spermatid and only remains of tails (D inset). (Spermatids and giant cells are indicated by small arrows and arrow heads respectively; H&E staining, ×100).
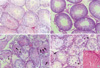
Fig. 2
Histology of the testis in rats treated with cadmium 4 mg/kg: diffuse and marked ischemic necrosis of testis (A); necrosis of all seminiferous tubules and relatively well-preserved interstitial tissues with some inflammatory cells infiltrates (B). (H&E staining, ×400).
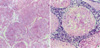
Fig. 3
DNA histograms of testicular cell suspension from flow cytometric analysis in rats receiving cadmium 0 mg/kg (A), 1 mg/kg (B) and 2 mg/kg (C). Disclosea peaks are for haploid elongated spermatid (M1), haploid round spermatid (M2), diploid germ cells and non-germ cells (M3), tetraploid germ cells (M5). Corresponding histograms for rats receiving α-tocopherol in addition are show in (D), (E) and (F).

Table 1
Length, width and weight of rat testes with diameters of seminiferous tubules (DST) (mean±SD)
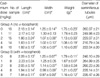
Table 2
Flow cytometric analysis (Mean±SD) of rat testes to cadmium without (Group A) and with α-tocopherol (Group B) pre-treatment
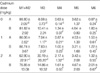
Within-group differences by one-way ANOVA are represented by * and † for p<0.01 and p<0.05 respectively. Significant pairwise comparison by Bonferroni t-procedure is marked by ‡ for p<0.01. Peaks are for haploid elongated spermatid (M1), haploid round spermatid (M2), diploid germ cells and non-germ cells (M3), tetraploid germ cells (M5).
References
1. Page AL, Chang AC. Foulkes EC, editor. Cadmium in the environment and its entry into terrestrial food chain crops. Handbook of Experimental Pharmacology. 1986. Berlin: Springer-Verlag;33–74.

2. Itokawa Y, Abe T, Tanaka S. Bone changes in experimental chronic cadmium poisoning: radiological and biological approaches. Arch Environ Health. 1973. 26:241–244.
3. Din WS, Frazier JM. Protective effect of metallothionein on cadmium toxicity in isolated rat hepatocytes. Biochem J. 1985. 230:395–402.

4. Axelsson B, Piscator M. Renal damage after prolonged exposure to cadmium. An experimental study. Arch Environ Health. 1966. 12:360–373.
5. Verschoor M. Renal function of workers with low-level cadmium exposure. Scand J Work Environ Health. 1987. 13:232–238.

6. Wong KL, Klaassen CD. Neurotoxic effects of cadmium in young rats. Toxicol Appl Pharmacol. 1982. 63:330–337.
7. Parizek J. The effect of a subcutaneous injection of cadmium salts on the ovaries of adult rats in persistent oestrus. J Reprod Fertil. 1968. 17:559–562.

8. Benoff S, Jacob A, Hurley IR. Male infertility and environmental exposure to lead and cadmium. Human Reproduction Update. 2000. 6:107–121.

9. Koizumi T, Li ZG. Role of oxidative stress in single-dose, cadmium-induced testicular cancer. J Toxicol Environ Health. 1992. 37:25–36.

10. Jamall IS, Smith JC. Effects of cadmium on glutathione peroxidase, superoxide dismutase, and lipid peroxidation in the rat heart: a possible mechanism of cadmium cardiotoxicity. Toxicol Appl Pharmacol. 1985. 80:33–42.

11. Manca D. In vitro and in vivo responses of rat tissues to cadmium-induced cadmiuminduced lipid peroxidation. Bull Environ Contam Toxicol. 1991. 46:929–936.
12. Hussain T, Shukla GS, Chandra SV. Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: in vivo and in vitro studies. Pharmacol Toxicol. 1987. 60:355–358.
13. Sikka SC, Rajasekaran M, Hellstrom WJ. Role of oxidative stress and antioxidants in male infertility. J Androl. 1995. 16:464–481.
14. Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987. 8:338–348.

15. Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989. 41:183–197.
16. Palamanda JR, Kehrer JP. Involvement of vitamin E and protein thiols in the inhibition of microsomal lipid peroxidation by glutathione. Lipids. 1993. 28:427–431.

17. Ernster L, Forsmark P, Nordenbrand K. The mode of action of lipid-soluble antioxidants in biological membranes: relationship between the effects of ubiquinol and vitamin E as inhibitors of lipid peroxidation in submitochondrial particles. Biofactors. 1992. 3:241–248.

18. Zante J, Schuman J, Gohde W, Hacker U. DNA-fluorometry of mammalian sperm. Histochemistry. 1977. 54:1–7.

19. Sharpe RM. Knobil E, Neill JD, editors. Regulation of spermatogenesis. The Physiology of Reproduction. 1994. New York: Raven Press;1363–1434.
20. Clermont Y. Quantitative analysis of spermatogenesis of the rat: a revised model for the renewal of spermatogonia. Am J Androl. 1962. 2:37–58.

21. Roosen-Runge EC. Germinal cell loss in normal metazoan spermatogenesis. J Reprod fertil. 1973. 35:339–348.
22. De Rooij G, Lok D. Regulation of the density of spermatogonia in the seminiferous epithelium of the Chinese hamster: II. Differentiating spermatogonia. Anat Rec. 1987. 217:131–136.

23. Russell LD, Alger LE, Nequin LG. Hormonal control of pubertal spermatogenesis. Endocrinology. 1987. 120:1615–1632.

24. Richburg JH, Boekelheide K. Mono-(2-ethylhexyl) phthalate rapidly alters both Sertoli cell vimentin filaments and germ cell apoptosis in young rat testes. Toxicol Appl Pharmacol. 1996. 137:42–50.

25. Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991. 113:689–699.

26. Miraglia SM, Hayashi H. Histomorphometry of immature rat testis after heating. J Morphol. 1993. 217:65–74.

27. Kwon KS, Cho KJ, Yang EJ. The study of anatomical and biochemical effects of cadmium on the testis in rats. Dev Reprod. 1997. 1:125–132. [in Korean].
28. Srinivas M, Agarwala S, Gupta SD, Das SN, Jha P, Misro MM, Mitra DK. Effect if cyclosporine on fertility in male rats. Pediatr Surg Int. 1998. 13:388–391.
29. Hacker U, Schumann J, Gohde W. Effects of acute gamma-irradiation on spermatogenesis as revealed by flow cytometry. Acta Radiol Oncol. 1980. 19:361–368.

30. Suleiman SA, Elamin AM, Zaki ZM, El-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl. 1996. 17:530–537.
31. Therond P, Auger J, Legrand A, Jouannet P. Alpha-tocopherol in human spermatozoa and seminal plasma: relationships with motility, antioxidant enzymes and leukocytes. Mol Hum Reprod. 1996. 2:739–744.
32. Askari HA, Check JH, Peymer N, Bollendrof A. Effect of natural antioxidants tocopherol and ascorbic acids in maintenance of sperm activity during freeze-thaw process. Arch Androl. 1994. 33:11–15.

33. Ollero M, Blanco TM, Lopez-Perez MJ, Cebrian Perez JA. Surface changes associated with ram sperm cryopreservation revealed by counter-current distribution in an aqueous two-phase system. Effect of different cryoprotectants. J Chromatogr B Biomed Appl. 1996. 680:157–164.

34. Beconi MT, Francia CR, Mora NG, Affranchino MA. Influence of antioxidants on SOD activity in bovine sperm. Biochem Int. 1991. 23:545–553.
35. O'Flaherty C, Beconi M, Beorlegui N. Effect of natural antioxidants, superoxide dismutase and hydrogen peroxide on capacitation of frozen-thawed bull spermatozoa. Andrologia. 1997. 29:269–275.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download