Abstract
We aimed to compare the CT findings of nontuberculous mycobacterial pulmonary diseases caused by Mycobacterium avium-intracellulare complex (MAC) and Mycobacterium abscessus. Two chest radiologists analyzed retrospectively the thin-section CT findings of 51 patients with MAC and 36 with M. abscessus infection in terms of patterns and forms of lung lesions. No significant difference was found between MAC and M. abscessus infection in the presence of small nodules, tree-in-bud pattern, and bronchiectasis. However, lobar volume decrease (p=0.001), nodule (p=0.018), airspace consolidation (p=0.047) and thin-walled cavity (p=0.009) were more frequently observed in MAC infection. The upper lobe cavitary form was more frequent in the MAC (19 of 51 patients, 37%) group than M. abscessus (5 of 36, 14%) (p=0.029), whereas the nodular bronchiectatic form was more frequent in the M. abscessus group ([29 of 36, 81%] vs. [27 of 51, 53%] in MAC) (p=0.012). In conclusion, there is considerable overlap in common CT findings of MAC and M. abscessus pulmonary infection; however, lobar volume loss, nodule, airspace consolidation, and thin-walled cavity are more frequently seen in MAC than M. abscessus infection.
Nontuberculous mycobacterial (NTM) organisms are widely distributed in the environment and the frequency of NTM pulmonary disease has been reported to be increasing (1, 2). NTM species are divided into slow-growing and rapid-growing species by the Runyon classification (2). Representatives of the slow-growing species include; M. avium, M. intracellulare (Mycobacterium avium-intracellulare complex: MAC) and M. kansasii, while those of the rapid-growing species include; M. abscessus, M. fortuitum and M. chelonae. In the United States, MAC species accounts for more than two thirds of NTM pulmonary diseases caused by slow-growing organisms and M. abscessus accounts for most pulmonary diseases caused by rapid-growing organisms (3-5). In Korea, MAC and M. abscessus represent the common pathogen in NTM lung disease and M. kansasii is relatively uncommon (6-10).
The CT findings of MAC pulmonary disease have been reported and are well known (11-14). Recently CT findings of M. abscessus were reported (15). According to these reports, CT findings of pulmonary diseases caused by the organisms of two species are mainly bronchiolitis of tree-in-bud pattern and bronchiectasis (11-16). However, no study has directly compared the CT findings of the pulmonary diseases caused by these two pathogens. Moreover, the treatment of pulmonary disease caused by these organisms is totally different. The pulmonary disease caused by M. abscessus needs intravenous antimycobacterial chemotherapy necessitating hospitalization for several weeks, whereas that caused by MAC needs oral antimycobacterial therapy on outpatient basis (2, 5). Therefore, CT differentiation between the diseases caused by these species before definite diagnosis by microbiological culture is important for treatment. In this study, we aimed to analyze the thinsection CT findings of pulmonary NTM diseases caused by MAC and M. abscessus and to compare the findings of the two groups.
Between July 1999 and March 2004, we diagnosed a total of 105 patients with NTM pulmonary diseases and who underwent chest CT. Of these 105 patients, 51 patients had MAC infection; 25 men with mean ages, 66 yr (range; 46-87 yr) and 26 women with mean ages, 56 yr (range; 25 to 74 yr). Thirty-six had M. abscessus infection; 9 men with mean ages, 51 yr (range; 21-75 yr) and 27 women with mean ages, 56 yr (range; 32-77 yr). Of the remaining 18 patients, seven had M. kansasii infection, six M. fortuitum complex, two M. chelonae, two M. szulgai, and one M. celatum.
We retrospectively reviewed the CT findings of these 87 patients with MAC and M. abscessus infection. The 87 patients met the diagnostic criteria of the American Thoracic Society inclusion criteria for NTM pulmonary disease: 1) clinical signs and symptoms; 2) compatible radiologic findings; and 3) multiple positive sputum cultures, recovery of NTM in large amounts on microbiologic smears and cultures of bronchoscopic samples, or compatible histopathology with a positive NTM culture (2).
The interval between the isolation of MAC and initial CT examination ranged from 0 to 328 days (mean, 22 days; median, 0 day), whereas that between the isolation of M. abscessus and initial CT examination ranged from 0 to 173 days (mean, 10 days; median, 0 day).
All CT examinations were obtained using a Light Speed Advantage Q/xi Scanner (General Electric Medical Systems, Milwaukee, WI, U.S.A.). None of the patients were administered an intravenous injection of contrast medium. All CT data were reconstructed using a bone algorithm. Helical volumetric scan data, using multidetector-row CT (120 kVp, 70 mA, 2.5-mm collimation, and pitch of 0.875), were obtained through the thorax. Data were reconstructed with 2.5-mm thickness for transaxial images. The scan data were directly displayed on monitors (four monitors, 1,536×2,048 image matrices, 8-bit viewable gray scale, and 60-ft-lambert luminescence) of a picture archiving and communication system (PACS) (Centricity 1.0, General Electric Medical Systems Integrated Imaging Solutions, MT. Prospect, IL, U.S.A.). On the monitors, both mediastinal (window width, 400 H; window level, 20 H) and lung (window width, 1,500 H; window level, -700 H) window images were available for analysis.
Two chest radiologists (T.S.K. and M.J.C. with seven and six years of experience, respectively), who were unaware of any clinical information, except that the patients had NTM pulmonary disease, assessed the CT images together, and reached final decisions by consensus. The presence of the patterns of parenchymal abnormalities in each lobe (six lobes: the right upper lobe, right middle lobe, right lower lobe, left upper lobe, lingual segment, and left lower lobe) in each patient was recorded. The evaluated patterns of parenchymal abnormalities included well-defined small nodules (less than 10 mm in diameter), branching centrilobular nodules (tree-in-bud pattern), nodules of 10-30 mm in diameter, airspace consolidation, thin-walled (less than 5 mm in thickness) cavities, bronchiectasis, and volume decrease. Regardless of size or distribution [lobular (0.5-3.0 cm in diameter and polygonal), segmental (pleura-based and polygonal or truncated-cone appearance), or peribronchial (along the bronchovascular bundles)], all forms of consolidation were grouped into airspace consolidation. When a nodule contained cavity, it was designated a cavitary nodule, and when consolidation contained cavity, it was designated a cavitary consolidation. In addition, the presence of mediastinal or hilar lymph node enlargement and pleural effusion or thickening was recorded.
The laterality (unilateral or bilateral) and the locations of lung lesions were also analyzed. A total of 306 lung lobes in 51 patients (six lobes per patient; the lingual segment was taken as a separate lobe) with MAC infection and 216 lobes in 36 patients with M. abscessus infection were evaluated for the presence of lung lesions. Each lung lobe was evaluated with regard to the presence or absence of each parenchymal abnormality.
After analyzing the pattern and distribution of parenchymal abnormalities at CT, diseases were classified into three forms: upper lobe cavitary, nodular bronchiectatic, and an unclassifiable form. The upper lobe cavitary form was defined when thin-walled cavity (or cavities) were present in the upper lobes with findings of emphysematous change in the lower lung zones with or without volume decrease of the upper lobes and apical pleural thickening (2) (Fig. 1). The nodular bronchiectatic form was defined when bilateral bronchiectasis and bronchiolitis were present, irrespective of the presence of cavities in both lungs. However, in this form, there was neither upper lobar volume loss nor emphysematous change in the remaining lungs (2) (Fig. 2). When the disease did not belong to either the upper lobe cavitary or the nodular bronchiectatic form, it was deemed unclassifiable. In this form, multi-focal lobular or segmental consolidation or consolidation along the bronchovascular bundles was seen (Fig. 3).
We evaluated difference in male to female ratios in MAC and M. abscessus infections using the chi-square test. The difference in forms (upper lobe cavitary, nodular bronchiectatic and unclassifiable) in MAC and M. abscessus infection was compared using the Fisher's exact test. The significance of differences in the presence of each pattern of parenchymal abnormality in the MAC and M. abscessus pulmonary diseases was tested using the Mann-Whitney test. We tested difference in the extent of involvement (i.e., the number of involved lobes) of each pattern of parenchymal abnormality between the two groups using the chi-square test.
Women were more frequently affected than men (p=0.003) in M. abscessus infection, whereas there was no sex difference in MAC infection (p=0.889).
Nineteen (37%) of 51 MAC patients had the upper lobe cavitary form, 27 (53%) the nodular bronchiectatic form, and 5 (10%) the unclassifiable form. Five of 36 M. abscessus patients (14%) had the upper lobe cavitary form, 29 (81%) the nodular bronchiectatic form, and 2 (5%) the unclassifiable form. The upper lobe cavitary form was relatively more frequent in the MAC group than M. abscessus (p=0.029), whereas the nodular bronchiectatic form was more frequent in the M. abscessus group (p=0.012).
The laterality and distribution of each type of parenchymal abnormality (e.g., bronchiectasis, small nodules, and tree-inbud pattern) are summarized in Table 1. The results and comparisons with regard to the presence of each parenchymal abnormality and the extent of each parenchymal lesion on a lobe basis in the MAC and M. abscessus groups are summarized in Table 2.
CT findings in MAC and M. abscessus groups overlapped. The commonest CT findings were bronchiectasis (48 of 51 patients [94%] in MAC infection and 33 of 36 [92%] in M. abscessus infection), small nodules (34 of 51 patients [67%] in MAC infection and 30 of 36 [83%] in M. abscessus infection), and tree-in-bud pattern (33 of 51 patients [65%] in MAC infection and 30 of 36 [83%] in M. abscessus infection) (Fig. 1, 2). No significant difference was observed between MAC and M. abscessus groups in the presence of parenchymal abnormalities on person basis except for lobar volume decrease (p=0.001), nodule (p=0.018), airspace consolidation (p=0.047) (Fig. 3) and a thin-walled cavity (p=0.009) (Fig. 4). These three abnormalities were more frequently seen in the MAC group (Table 2).
With regard to the extent of lobe involvement for each parenchymal abnormality, tree-in-bud pattern (p=0.013) were observed in more lobes in M. abscessus pulmonary disease than in MAC, whereas lobar volume loss (p<0.001), nodule (p<0.001), cavitary nodules (p=0.002), airspace consolidation (p=0.002) and thin-walled cavity (p=0.014) were observed in more lobes in MAC than M. abscessus (Table 2).
In MAC pulmonary disease, two distinct radiologic subtypes, the upper lobe cavitary form and the nodular bronchiectatic form, have been noted (2). The former, the traditional and most widely known presentation of MAC pulmonary disease, is usually seen in white, middle-aged or elderly men who smoke or abuse alcohol. Underlying disorders commonly include chronic obstructive pulmonary disease, previous tuberculosis, and silicosis. The second clinical presentation, the so-called nodular bronchiectatic form, was recognized more recently (1), and occurs predominantly in non-smoking middle-aged or elderly women who also present with a chronic productive cough. Interestingly, previous or underlying lung disease has not been noted in these patients (17, 18). However, because CT findings of the upper lobe cavity and nodular bronchiectasis are frequently observed in the same patient (19) and CT findings of predominantly airspace pattern are seen in patients with NTM pulmonary disease, it is sometimes difficult to divide MAC pulmonary disease into two different forms of disease based on the above forms of the disease, and therefore, we introduced an unclassifiable disease form. In our study, all three forms of disease (upper lobe cavitary, nodular bronchiectatic and unclassifiable forms) were observed in MAC and in M. abscessus infection, although the nodular bronchiectatic form was dominant in both. Moreover, the nodular bronchiectatic form was significantly more frequent in patients with M. abscessus infection.
During an analysis of CT findings of MAC pulmonary disease in 55 patients, in which two forms (upper lobe cavitary and nodular bronchiectatic) of disease were placed together, Lynch et al. (13) noted nodules (49 of 55 patients, 89%), airspace consolidation (44, 80%), bronchiectasis (40, 73%), and cavities (66%) are predominant findings. In their study, the presence of widespread bronchiectasis was found to allow the differentiation of MAC infection from pulmonary M. tuberculosis infection. In another similar study, Obayashi et al. (14) analyzed the CT findings of MAC pulmonary disease in 25 patients (both lungs in each patient were divided into 10 zones), and also combined the two forms of disease. Centrilobular small nodules (167 of 250 zones), bronchiectasis (133 of 250 zones), nodules (81 of 250 zones), airspace consolidation (30 of 250 zones), and cavity (11 of 250 zones) were common CT findings. Therefore, in MAC pulmonary disease, airspace consolidation and cavity are common CT findings in addition to bronchiectasis and centrilobular nodules (tree-in-bud pattern). These observations were further corroborated by our study. In addition, the present study showed a significantly higher frequency of airspace consolidation and thin-walled cavity in MAC than M. abscessus pulmonary disease (Table 2). Moreover, lobar volume decrease especially in the right middle lobe and lingular segment of the left upper lobe was observed more frequently in MAC infection than M. abscessus infection (Table 1, 2), in keeping with the results of previous reports (20, 21).
The reported CT findings of M. abscessus pulmonary disease are bilateral multi-focal bronchiectasis, bronchiolitis [small nodules (<5 mm) and branching centrilobular lesions (so-called tree-in-bud pattern)], and focal areas of consolidation (15). In the current study, bronchiectasis, small nodules and tree-in-bud patterns were the dominant findings of this disease. Although cavitary nodule, lobular consolidation, thin-walled cavity, and peribronchial consolidation were also seen in M. abscessus infection, they were present in less than 30% of patients and occurred less frequently than in MAC infection.
When comparing the CT findings of MAC and M. abscessus infection, their common findings were bilateral small nodules, tree-in-bud pattern, and bronchiectasis. No significant difference was observed between MAC and M. abscessus groups in the presence of these three parenchymal abnormalities on person basis except for a lobar volume decrease, nodule, airspace consolidation and a thin-walled cavitary. The latter four abnormalities were more frequently seen in the MAC group. With regard to the extent of lobe involvement for each parenchymal abnormality, tree-in-bud pattern was observed in more lobes in M. abscessus disease than in MAC disease, whereas lobar volume loss, nodule, cavitary nodules, airspace consolidation and thin-walled cavity were observed in more lobes in cases of MAC disease.
The relative applicability of the CT distinction between patients with MAC and M. abscessus infection needs to be elaborated. Although only one third or one half of patients with bilateral bronchiolitis and bronchiectasis prove to have NTM pulmonary infection, thin-section CT findings of bronchiectasis plus bronchiolitis involving more than 5 lobes, especially when associated with lobular consolidation or a cavity, are highly suggestive of NTM pulmonary infection (22). In this condition, the distinction may be important. Moreover, when sputum or bronchial washing fluid shows positive acid-fast bacilli staining or transbronchial lung biopsy specimen demonstrates tissue acid-fast bacilli staining positivity or chronic granulomatous inflammation, the above-mentioned CT findings strongly suggest NTM pulmonary infection. In this condition, the CT distinction is helpful for guiding treatment and for predicting the prognosis of patients, because the treatment and prognosis of NTM pulmonary infection differ according to etiologic organisms.
Patients with pulmonary disease caused by MAC are treated with combined regimen of clarithromycin or azithromycin, refampin or rifabutin, and ethambutol on an outpatient basis and with a relatively good response (2). In contrast with MAC infection, patients with M. abscessus infection need hospitalization for treatment with administration of parenteral antibiotics such as amikacin and cefoxitin for several weeks. Unfortunately, however, M. abscessus pulmonary disease is extremely difficult to eradicate, and it responds best to surgical resection of localized disease (2, 5). Therefore, CT differentiation between the diseases caused by these species before definite diagnosis with microbiological culture is important, taking into consideration of treatment options and prognoses.
In conclusion, there is considerable overlap in CT findings between MAC and M. abscessus pulmonary diseases and nodular bronchiectatic form with bilateral tree-in-bud pattern and bronchiectasis is commoner for both infections than upper lobe cavitary form. However, the upper lobe cavitary form is relatively more frequent in MAC infection. And patterns of lobar volume loss, nodule, airspace consolidation, and thin-walled cavity are more frequently associated with MAC infection. Whereas there is no sex difference in MAC infection, women are more frequently affected than men in M. abscessus infection.
Figures and Tables
Fig. 1
A 66-yr-old man with M. avium-intracellulare complex pulmonary disease. (A) Transaxial thin-section (2.5-mm thickness) CT scan obtained at level of great vessels shows multiple large thin-walled cavities in both lung apices. Also note several small nodules (arrows) in right lung. (B) CT (2.5-mm thickness) scan obtained at level of suprahepatic inferior vena cava shows multiple small nodules and branching centrilobular nodules, so-called tree-in-bud pattern (arrows), in lingular segment of left upper lobe and left lower lobe. Small nodule (arrowhead) is also seen in right middle lobe.

Fig. 2
A 45-yr-old woman with M. abscessus pulmonary disease. (A) Transaxial thin-section (2.5-mm thickness) CT scan obtained at level of proximal lower lobar bronchus shows bronchiectasis (arrows) and multiple small nodules and branching centrilobular nodules, so-called tree-in-bud pattern (arrowheads) in right lung. (B) CT (2.5-mm thickness) scan obtained at level of basal trunk shows bronchiectasis (arrows) and multiple small nodules and branching centrilobular nodules, so-called tree-in-bud pattern (arrowheads) in both lungs.
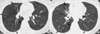
Fig. 3
A 52-yr-old woman with M. avium-intracellulare complex pulmonary infection. (A) Transaxial thin-section (2.5-mm thickness) CT scan obtained at level of great vessels shows subsegmental consolidation with open bronchus sign in right upper lobe (arrows). (B) CT (2.5-mm thickness) scan obtained at level of right upper lobar bronchus shows airspace consolidation with surrounding ground-glass opacity (arrows) along bronchovascular bundles in anterior segments of both upper lobes. Also note small nodules in right upper lobe. (C), CT (2.5-mm thickness) scan obtained at lung base shows lobular consolidation in left lower lobe (arrows). Also note lesion of tree-in-bud pattern (arrowhead) in left lower lobe and small nodules in right middle lobe.
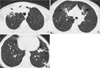
Fig. 4
A 69-yr-old woman with M. avium-intracellulare complex pulmonary disease. Transaxial thin-section (2.5-mm thickness) CT scan obtained at level of bronchus intermedius shows multiple cavitary nodules (arrowheads) in both lungs. Also note small nodules (arrows) and bronchiectasis (white arrows).
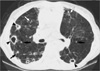
Table 1
Laterality and distribution of parenchymal lesions in patients with MAC (n=51) and M. abscessus (n=36) disease

MAC, Mycobacterium avium-intracellulare complex; Uni, unilateral; Bi, bilateral; RUL, right upper lobe; RML, ight middle lobe; RLL, right lower lobe; LUL, left upper lobe; Li, lingular segment; LLL, left lower lobe; +, number of patients; *, number of involved lobe (of total 306 lobes in MAC and 216 lobes in M. abscessus infection).
References
1. Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med. 2002. 23:553–567.
2. American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med. 1997. 156:S1–S25.
3. O'Brien RJ, Geiter LJ, Snider DE Jr. The epidemiology of nontuberculous mycobacterial disease in the United States: results from a national survey. Am Rev Respir Dis. 1987. 135:1007–1014.
4. Brown-Elliott BA, Wallace RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmented rapidly growing mycobacteria. Clin Microbiol Rev. 2002. 15:716–746.
5. Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria: an analysis of 154 patients. Am Rev Respir Dis. 1993. 147:1271–1278.

6. Lew WJ, Ahn DI, Yoon YJ, Cho JS, Kwon DW, Kim SJ, Hong YP. Clinical experience on mycobacterial diseases other than tuberculosis. Tuberc Respir Dis. 1992. 39:425–432.
7. Bai GH, Park KS, Kim SJ. Clinically isolated mycobacteria other than Mycobacterium tuberculosis from 1980 to 1990 in Korea. J Korean Soc Microbiol. 1993. 28:1–5.
8. Korean Academy of Tuberculosis and Respiratory Disease. National survey of mycobacterial diseases other than tuberculosis in Korea. Tuberc Respir Dis. 1995. 42:277–294.
9. Koh WJ, Kwon OJ, Ham HS, Suh GY, Chung MP, Kim H, Han D, Kim TS, Lee KS, Lee NY, Park EM, Park YK, Bai GH. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens. Korean J Intern Med. 2003. 65:10–21.
10. Koh WJ, Kwon OJ, Suh GY, Chung MP, Kim H, Lee NY, Kim TS, Lee KS, Park EM, Park YK, Bai GH. A case report of three patients with nontuberculous mycobacterial pulmonary disease caused by Mycobacterium kansasii. Tuberc Respir Dis. 2003. 54:459–466.
11. Moore EH. Atypical mycobacterial infection in the lung: CT appearance. Radiology. 1993. 187:777–782.

12. Primack SL, Logan PM, Hartman TE, Lee KS, Muller NL. Pulmonary tuberculosis and Mycobacterium avium-intracellulare: a comparison of CT findings. Radiology. 1995. 194:413–417.

13. Lynch DA, Simone PM, Fox MA, Bucher BL, Heinig MJ. CT features of pulmonary Mycobacterium avium complex infection. J Comput Assist Tomogr. 1995. 19:353–360.

14. Obayashi Y, Fujita J, Suemitsu I, Kamei T, Nii M, Takahara J. Successive follow-up of chest computed tomography in patients with Mycobacterium avium-intracellulare complex. Respir Med. 1999. 93:11–15.

15. Han D, Lee KS, Koh WJ, Yi CA, Kim TS, Kwon OJ. Radiographic and CT findings of nontuberculous mycobacterial pulmonary infection caused by Mycobacterium abscessus. AJR Am J Roentgenol. 2003. 181:513–517.

16. Jeong YJ, Lee KS, Koh WJ, Han J, Kim TS, Kwon OJ. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. Radiology. 2004. 231:880–886.

17. Prince DS, Peterson DD, Steiner RM, Gottlieb JE, Scott R, Israel HL, Figueroa WG, Fish JE. Infection with Mycobacterium avium complex in patients without predisposing condition. N Engl J Med. 1989. 321:863–868.
18. Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. Chest. 1992. 101:1605–1609.

19. Hollings NP, Wells AU, Wilson R, Hansell DM. Comparative appearances of non-tuberculous mycobacteria species: a CT study. Eur Radiol. 2002. 12:2211–2217.

20. Fujiuchi S, Matsumoto H, Yamazaki Y, Nakao S, Takahashi M, Satoh K, Takeda A, Okamoto K, Fujita Y, Fujikane T, Shimizu T. Analysis of chest CT in patients with Mycobacterium avium complex pulmonary disease. Respiration. 2003. 70:76–81.
21. Wittram C, Weisbrod GL. Mycobacterium avium complex lung disease in immunocompetent patients: radiography-CT correlation. Br J Radiol. 2002. 75:340–344.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download