Abstract
Occupational asthma is induced by many agents, including herbal materials, that are exposed in working places. Although there are a few case reports for occupational allergy induced by herbal materials, there is none for that induced by Wonji (Polygala tenuifolia). This study was conducted to evaluate clinical characteristics and immunologic mechanism of Wonji-induced asthma in a exposed-worker. A patient who complained of asthma and rhinitis symptoms, and who had worked in a herbal manufacturing factory for 8 yr, underwent a skin prick test with crude extract of Wonji under the impression of occupational asthma induced by the agent. The patient had a strong positive response to the extract on the skin prick test. Allergen bronchial challenge to the extract demonstrated a typical dual response. Serum specific IgE level to the extract was higher in the patient than in healthy controls, and ELISA inhibition test revealed complete inhibition of IgE binding with the extract, but no inhibition with Der p 2 or mugwort extracts. Six IgE binding components to the extract (10, 25, 28, 36, 50, and 90 kDa) were detected using SDS-PAGE and immunoblot analysis. These findings suggest that Polygala tenuifolia, a herbal material, can induce IgE-mediated bronchoconstriction in exposed workers.
There have been a few reported cases of occupational asthma induced by herbal materials. An IgE-mediated mechanism has been implicated in most cases including Sanyak (1), Chunkung (2), Banha (1-3), liquorice (4), and Brazil ginseng (5).
Polygala tenuifolia belongs to the Polygalaceae family and has been cultivated throughout East Asia, including Korea. Wonji is made from the root of P. tenuifolia and used as an expectorant and antipsychotic agent in Korean traditional medicine. To the best of our knowledge, Wonji-induced occupational asthma and rhinitis has not been reported yet, and its pathogenic mechanism remains unknown. This study is therefore the first report of occupational asthma and rhinitis induced by Wonji with an IgE-mediated mechanism.
A 45-yr-old male had complained of recurrent wheezing and dyspnea for 7 months. He had worked in a herbal manufacturing factory for 8 yr where he classified many kinds of herbal materials, including Wonji, to prepare for processing. He had experienced nasal symptoms including profuse rhinorrhea and sneezing 4 yr after starting work. These symptoms were aggravated when handling Wonji in the manufacturing process, but improved during holidays and vacations. He was a non-smoker, and had no relative with asthmatic symptoms. His serum total IgE level was 663 U/mL using fluorescent enzyme immunoassay, and the total eosinophil count in the blood was 578/µL. A skin prick test with 55 common aeroallergens showed immediate positive responses to Dermatophagoides pteronyssinus, Dermatophagoides farinae, tree pollens, and grass pollens. Methacholine bronchial challenge test revealed 20% decline of FEV1 at the methacholine concentration of 1.9 mg/mL.
Wonji was obtained from the patient's employer and was prepared as previously described (1). In brief, Wonji was cut into small pieces and extracted into phosphate-buffered saline (PBS; pH 7.5) 1:5 wt/vol, at 4℃ for 24 hr, followed by centrifugation at 15,000 rpm at 4℃ for 30 min. The supernatant was dialyzed (the cut-off molecular weight was 6 kDa) against 4L of normal saline at 4℃ for 48 hr and then used as crude extracts. The protein concentration of the extracts was determined by bicinchoninic acid (BCA) assay according to the manufacturer's instructions (Pierce, Rockford, IL, U.S.A.). The extract was used for the skin prick tests and specific bronchial challenge test.
One mL of normal saline was administered from a DeVilbiss 646 nebulizer (CS & M Instrument Co., Doylestown, PA, U.S.A.) connected to a dosimeter. The subject was asked to breathe the aerosol with tidal breathing, and this was followed by Wonji extract. The forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were measured using a spirometer (Multispiro-SX™, Irvine, CA, U.S.A.) before and 10 min after each inhalation, every 10 min during the first hour, and then every hours for the next 8 hr after the challenge.
The presence of specific IgE antibody to Wonji extract was determined by ELISA, as preciously described (6). In brief, microtiter plates (NUNC; immunoplate, Roskilde, Denmark) were coated with 50 µL of Wonji extract (100 µg/mL), and then incubated with 50 µL of either the patient's serum, or undiluted sera from five asthmatics who showed negative skin prick test responses to common aeroallergens as well as Wonji extract. After washing, the immunoplate was incubated with 1:1,000 vol/vol biotin-labeled goat anti-human IgE antibody (Sigma, St. Louis, MO, U.S.A.) followed by incubation with 1:1,000 vol/vol streptavidin-peroxidase (Sigma). After washing, 75 µL of TMB solution, 3,3',5,5'-tetramethylbenzidine, one tablet in 10 mL of phosphate citrate buffer containing 2 µL of 30% hydrogen peroxide was added as substrate, and 75 µL of 2.5 N H2SO4 was added to stop the reaction 5 min later. A calorimetric reaction was measured by the absorbency at 450 nm on an ELISA reader. All assays were performed in triplicate.
Competitive ELISA inhibition tests were performed to determine the specificity of IgE binding to a Wonji antigen. In brief, 50 µL of the patient's serum were preincubated with Wonji extract and Der p 2, mugwort extracts for 1 hr at room temperature. The mixture was then incubated on a Wonji-coated microtiter plate for 2 hr. The same steps were then followed as for ELISA. After studying the control samples, in which equal volumes of PBS were preincubated instead of inhibitors, the inhibition of the specific IgE binding was expressed as: 100-(absorbance of samples preincubated with allergens/absorbance of samples preincubated with PBS)×100 (%).
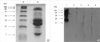
The protein composition pattern and specific IgE binding components of Wonji were analyzed by SDS-PAGE and immunoblot analysis by using the patient's serum. In brief, 25 µg of Wonji extracts were loaded and separated by 12% SDS-PAGE. After electrophoresis, the gel was stained with Coomassie Brilliant Blue R-250 solution (Bio-Rad, Hercules, CA, U.S.A.) and analyzed. To identify the specific IgE binding components of Wonji extracts, immunoblot analysis was performed with the asthmatic patient's serum. After separating the proteins by SDS-PAGE, they were electrophoretically transferred from the gel to a nitrocellulose membrane in a Bio-Rad Trans-Blot system. Blocking was done by incubation in a solution of 10% non-fat dried milk in 0.05% TBS-T buffer, pH 7.5 for 1 hr at room temperature. The nitrocellulose membrane was then washed, cut into strips and separately incubated overnight at 4℃, with the patient's serum which had been diluted 1:10 with the blocking solution. The membrane was then washed and incubated with goat anti-human IgE conjugated HRP (Sigma, St. Louis, MO, U.S.A.), in the presence of blocking solution, for 1 hr at room temperature. After further washing, the membrane was incubated in SuperSignalWest Pico chemiluminescent substrate (Pierce, Rockford, IL, U.S.A.) for 5 min. Fluorescence signals were detected by autoradiography using the Kodak Biomax Light ML film (Eastman Kodak Company, Rochester, NY, U.S.A.).
The mean size of the wheal formed by skin prick test with 1:10, 1:100, and 1:1,000 dilutions of Wonji extract, and 1 mg/mL of histamine were 6.5, 3, 2.5 and 2.5 mm respectively.
There was no significant change in FEV1 after inhalation of 1:1,000,000, 1:100,000 and 1:10,000 dilutions of Wonji extract, but the patient complained of nasal symptoms, such as sneezing and rhinorrhea, during that period. Interestingly, a dual asthmatic reaction was noted after inhalation of 1:1,000 dilution of extract, as shown in Fig. 1.
When compared with control sera, specific IgE binding to Wonji extract was detectable in the patient's serum (Fig. 2). Specific IgE binding to Wonji extract was completely inhibited by the addition of 1:1,000 dilution of Wonji extract, although not by the addition of Der p 2 or mugwort pollen (Fig. 3).
To determine the IgE-producing protein components from the Wonji extract, the extract was analyzed by 12% SDS-PAGE (Fig. 4A). From immunoblot analysis, six protein components from Wonji extract (10, 25, 28, 36, 50, and 90 kDa) were specifically bound to IgE from the patient's serum, although they were not bound with the control sera (Fig. 4B).
Occupational asthma can be defined as asthma that is induced by inhaled agents encountered in the workplace. The diagnosis of occupational asthma rests, firstly, on the identification of variable airflow obstruction; secondly, on exposure to a sensitizing agent at work and thirdly, on a causal relationship between them (7). The identification of specific IgE antibodies in a patient in whom this is suspected provides useful evidence to support the diagnosis. High antibody levels with a characteristic history are often taken to be sufficient evidence for a diagnosis of occupational asthma (8). In the case of a high molecular weight agent, the combination of a positive skin test and airway hyperresponsiveness has an 80% probability of predicting occupational asthma (9, 10). This study is the first case report of Wonji-induced occupational asthma and rhinitis.
In terms of immunopathogenesis of Wonji-induced asthma, IgE-mediated hypersensitivity is likely to be responsible for the respiratory symptoms. Both immunologic and non-immunologic mechanisms are suggested to be involved in cases of occupational asthma due to the high molecular weight agents. The immunologic mechanism can have either an IgE or a non-IgE reaction. High molecular weight agents, such as plant and animal proteins, enzymes, and large carbohydrate molecules, can induce IgE-mediated occupational asthma (11). However, the effect of other immunologic mechanisms on the occupational asthma induced by large molecules has not been clearly established. In this study, the patient had a strong positive response to Wonji antigen, which was prepared by the dialysis step through the membrane of 6,000-Da cut-off. ELISA inhibition test confirmed the specificity of IgE binding to the extract. This finding suggests that high molecular weight proteins within the Wonji extract may induce IgE-mediated bronchoconstriction in an exposed worker.
There have been several reports of atopy playing an important role in the development of IgE sensitization to occupational allergens with high molecular weight (12-16). The patient of this study had atopy and showed positive responses to Dermatophagoides pteronyssinus, Dermatophagoides farinae, tree pollens, and grass pollens. However, he had never experienced any nasal or respiratory symptoms before being employed at his workplace. This finding suggests that atopy may be a risk factor for the development of asthma induced by herbal medicine. Taken together, the results of this study suggest that Wonji-derived allergens can induce occupational asthma and rhinitis with an IgE-medicated mechanism in exposed workers.
Figures and Tables
References
1. Park HS, Kim MJ, Moon HB. Occupational asthma caused by two herb materials, Dioscorea batatas and Pinellia ternata. Clin Exp Allergy. 1994; 24:575–581.

2. Lee SK, Cho HK, Cho SH, Kim SS, Nahm DH, Park HS. Occupational asthma and rhinitis caused by multiple herbal agents in a pharmacist. Ann Allergy Asthma Immunol. 2001; 86:469–474.

3. Kim SH, Jeong H, Kim YK, Cho SH, Min KU, Kim YY. IgE-mediated occupational asthma induced by herbal medicine, Banha (Pinellia ternate). Clin Exp Allergy. 2001; 31:779–781.
4. Cartier A, Malo JL, Labrecque M. Occupational asthma due to liquorice roots. Allergy. 2002; 57:863.

5. Subiza J, Subiza JL, Escribano PM, Hinojosa M, Garcia R, Jerez M, Subiza E. Occupational asthma caused by Brazil ginseng dust. J Allergy Clin Immunol. 1991; 88:731–736.

6. Kim YK, Son JW, Kim HY, Park HS, Lee MH, Cho SH, Min KU, Kim YY. New occupational allergen in citrus farmers: citrus red mite (Panonychus citri). Ann Allergy Asthma Immunol. 1999; 82:223–228.

7. Matte TD, Hoffman RE, Rosennman KD, Stanbury M. Surveillance of occupational asthma under the SENSOR model. Chest. 1990; 98:Suppl. 173S–178S.

8. Malo JL, Ghezzo H, L'Archeveque J, Lagier F, Perrin B, Cartier A. Is the clinical history a satisfactory means of diagnosing occupational asthma? Am Rev Respir Dis. 1991; 143:528–532.

9. Cockcroft DW, Murdock KY, Kirby J, Hargreave F. Prediction of airway responsiveness to allergen from skin sensitivity to allergen and airway responsiveness to histamine. Am Rev Respir Dis. 1987; 135:264–267.
10. Malo JL, Cartier A, L'Archeveque J, Ghezzo H, Lagier F, Trudeau C, Dolovich J. Prevalence of occupational asthma and immunologic sensitization to psyllium among health personnel in chronic care hospitals. Am Rev Respir Dis. 1990; 142:1359–1366.

11. Wild LG, Lopez M. Occupational asthma caused by high-molecular-weight substances. Immunol Allergy Clin North Am. 2003; 23:235–250.

12. Milton DK, Solomon GM, Rosiello RA, Herrick RF. Risk and incidence of asthma attributable to occupational exposure among HMO members. Am J Ind Med. 1998; 33:1–10.

13. Burge PS, Edge G, O'Brien IM, Harries MG, Hawkins R, Pepys J. Occupational asthma in a research centre breeding locusts. Clin Allergy. 1980; 10:355–363.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download