Abstract
The purpose of this study was to evaluate the early outcome of endovascular management in patients with iliofemoral deep venous thrombosis (DVT) due to iliac vein compression syndrome (IVCS) and protein C and/or S deficiency. Between September 2000 and January 2003, catheter-directed thrombolysis was performed in 11 patients with a diagnosis of acute iliofemoral DVT: 7 with protein C and/or S deficiency and 4 without protein C and/or S deficiency. After thrombolysis, the diagnosis of IVCS was confirmed in 6 patients: 4 with protein C and/or S deficiency and 2 without protein C and/or S deficiency. Further intervention consisted of angioplasty and stent placement was performed. Four patients with IVCS and protein C and/or S deficiency were included in this study. The immediate technical and clinical success rates were 100% in all 4 patients. There were no complications or clinically detectable pulmonary emboli. This initial experience suggests that endovascular management of iliofemoral DVT due to IVCS in patients with protein C and/or S deficiency is safe and effective.
Traditional therapy for iliofemoral deep venous thrombosis (DVT) has been systemic heparin followed by oral warfarin. Despite widespread use of this method, the results have been largely inadequate in terms of rapid resolution of symptoms, recanalization of long-segment venous occlusions, and long-term disability from chronic venous insufficiency (1-4). Thrombolysis for DVT, if performed soon after the onset of symptoms, has the potential to prevent damage to the deep valves, thus maintaining the integrity and preventing postthrombotic complications (4-9). During the past several years, catheter-directed thrombolysis and endovascular stents have been used to treat extensive lower extremity DVT, as an alternative to conservative anticoagulation therapy or surgical thrombectomy, in selected cases.
Thrombosis due to compression of the left common iliac vein by the overlying right common iliac artery was first characterized by May and Thurner in 1957 (10). With the advent of catheter-directed thrombolysis, iliac vein compression syndrome (IVCS) is emerging as a frequent finding at venography after iliofemoral vein thrombolysis. The term "hypercoagulable state" is generally used to denote any condition in which the normal balance between clotting and anticlotting mechanisms becomes altered in such a way that the patient is predisposed to thrombus formation. There are a number of conditions that can lead to a hypercoagulable state. Proteins C and S deficiencies are frequently described as causes of the hypercoagulable states (9, 11-19). Although instances of arterial thromboses have been reported, proteins C and S deficiencies have frequently been associated with venous thromboembolic events (9, 11-17).
The purpose of this study was to evaluate the early outcome of endovascular management in patients with iliofemoral DVT due to IVCS and protein C and/or S deficiency.
A total of consecutive 47 patients with DVT were seen in our department between September 2000 and January 2003. The diagnosis of DVT was confirmed by characteristic clinical, color Doppler ultrasound and/or conventional venographic findings. Of these, 10 patients (21.3%) were diagnosed with DVT associated with protein C and/or S deficiency. Hypercoagulability studies were done before thrombolysis and systemic anticoagulation therapy. Antigenic protein C and S levels were measured using human protein C/S 'NL' NANORID™ radial immunodiffusion kit (the Binding Site Ltd, Birmingham, U.K.), and the diagnosis of protein C and S deficiencies was confirmed if antigenic protein C and S levels were less than 60% of normal. In this study, 3 of the 10 patients with protein C and/or S deficiency did not receive catheter-directed thrombolysis: due to an isolated infrainguinal DVT (1) and a chronic DVT (2). Seven patients with acute iliofemoral DVT and protein C and/or S deficiency were treated with catheter-directed thrombolytic therapy, and 4 with the diagnosis of IVCS (compression of the left iliac vein by the right iliac artery) after thrombolysis were included in this study. There was no family history of DVT in all 4 patients. We did not performed family studies for protein C and/or S deficiency, because not all patients with these deficiencies will experience episodes of thrombosis and low levels of either factor by itself in an asymptomatic patient are not an indication for anticoagulation. Informed consent was obtained in all patients after the risks and benefits of treatment were fully explained.
Local catheter-directed thrombolytic therapy with urokinase (Green Cross Co., Yong In, Korea) was performed under the fluoroscopic guidance. Although this drug has recently (mid-1999) been withdrawn from clinical use in the U.S.A. and U.K. because of problems with purity in its manufacture and on the order of the Food and Drug Administration, we used urokinase as the thrombolytic agent in this study. With the patient in prone position, venous access was through the ipsilateral popliteal vein under the ultrasound guidance. Eight or 9-Fr vascular sheath (Check-flo; Cook, Bloomington, IN, U.S.A.) was placed and direct venography was obtained to estimate the severity and extent of the thrombosis above the popliteal vein. Before the insertion of infusion catheter for the thrombolysis, we aspirated part of the filling defect to curtail the duration and the amount of the thrombolytics with use of another long, smaller-bore vascular sheath. And then, a 5-Fr multi-side-hole infusion catheter was inserted through the sheath. Urokinase was reconstituted by using 500,000 IU in 100 mL of 0.9% NaCl and delivered in continuous low dose of 500-2,000 IU/min via thrombolysis catheter. Patients were systemically treated with heparin during the procedure through the side arm of the venous sheath after a loading bolus of 5,000 IU. Partial thromboplastin time values were obtained at every 4 hr after the start of thrombolytic therapy, and the heparin infusion rate was adjusted to maintain the partial thromboplastin time range with 50-90 sec. Follow-up venography was performed within 12 hr from the start of thrombolytic therapy. The position of the catheter was changed to facilitate effective thrombolysis. After thrombolysis, further intervention was performed if there was an underlying venous stenosis of greater than 50%. Intervention consisted of angioplasty and stent placement. The venous stenoses were predilated with an 8- or 10-mm-diameter balloon (Ultra-Thin Diamond; Boston Scientific Co., Watertown, MA, U.S.A.) before stent placement (Wallstent; Boston Scientific Co.). All stents were placed above the inguinal ligament.
Immediate technical success was defined as complete recanalization of the vein with less than 30% residual luminal (area) narrowing (4-6, 9). Immediate clinical success was defined as complete or partial resolution of low extremity pain and edema. After the procedure, all patients were given anticoagulants with a regimen of heparin and, later, orally administered life-long warfarin, adjusted to maintain a prothrombin time of 2.0 and followed up both clinically and by color Doppler ultrasound within one month.
Of the 47 patients, catheter-directed thrombolysis was performed in 11 patients (23.4%) with symptoms of less than 10 days' duration and a diagnosis of iliofemoral DVT confirmed by color Doppler ultrasound: 7 with protein C and/or S deficiency (2 right lower limbs and 5 left lower limbs) and 4 without protein C and/or S deficiency (4 left lower limbs). After thrombolysis, the diagnosis of IVCS was confirmed in 6 patients: 4 with protein C and/or S deficiency and 2 without protein C and/or S deficiency. Further intervention consisted of angioplasty and stent placement was performed.
The 4 consecutive patients with IVCS and protein C and/or S deficiency included in this study were 1 man and 3 women, aged 43-75 yr (mean, 62.3 yr). Clinical characteristics are summarized in Table 1. All patients underwent catheter-directed thrombolysis and endovascular stents without antecedent heparin therapy. The mean length of symptoms prior to the initiation of thrombolysis was 6.8 days, with a range of 5-10 days.
Overall, there were immediate technical and clinical success rates of 100% for treated limbs (Fig. 1). The average duration of therapy was 39.9 hr (range, 16.5-66.5 hr), and the average total urokinase dose was 2.8 million IU (range, 1.0 million to 4.7 million IU). There were no complications or clinically detectable pulmonary emboli. All 4 patients were asymptomatic at mean clinical follow-up of 11.5 months (range, 6-20 months). Serial color Doppler ultrasound in all 4 patients showed no evidence of recurrence of DVT and valvular insufficiency in the femoral and popliteal vein segments, using both proximal compression and Valsalva maneuver.
Conventional treatment of iliofemoral DVT has typically consisted with systemic heparin therapy followed by anticoagulation with oral warfarin. However, with anticoagulation, eventual recanalization of the occluded vessel depends solely on the effectiveness of the patient's own fibrinolytic system. Anticoagulation does not protect the patient from the manifestations of post-thrombotic syndrome, which can appear months to years after the acute episode of DVT (3-8). Long-term studies in patients with iliofemoral DVT treated with anticoagulation alone have demonstrated that muscle pump function and valvular competency are severely compromised in approximately 95% of patients at 5-yr follow-up despite improvement in venous outflow (20). The therapeutic goals for treating the patient with acute DVT include prevention of pulmonary embolism, restoration of unobstructed blood flow through the thrombosed segment, prevention of recurrent thrombosis, and preservation of venous valve function (4). Considering that success in the achievement of these clinical goals will minimize the morbidity and mortality associated with DVT, catheter-directed thrombolysis is a potentially attractive form of therapy: (a) it delivers high concentrations of thrombolytic agents directly into the thrombus while minimizing the potential for a systemic fibrinolytic effect, (b) it provides the opportunity for the prompt restoration of venous patency and preservation of venous valve function. This therapy can potentially help prevent long-term sequelae of DVT with acceptable complication rates.
Reported clinical series show that lower extremity DVT occurs three to eight times more frequently on the left when compared to the right (10, 21-23). Virchow first described the anatomic relationship of the right common iliac artery to the left common iliac vein and postulated that the relationship can produce venous stasis, thus accounting for the increased frequency of left leg DVT. Thrombosis due to compression of the left common iliac vein by the overlying right common iliac artery was first characterized by May and Thurner in 1957 (10). May and Thurner examined 430 cadavers and found 19% to have changes at the junction of the left common iliac vein with the inferior vena cava, which they termed the "spur". In 1967, Cockett et al. reported the first clinical series of 57 patients presenting with acute iliofemoral DVT secondary to iliac vein compression and coined it the "IVCS" (24). However, it is well known that compression of the left common iliac vein by the right common iliac artery is a common normal finding during iliocaval venography and all patients with iliac vein compression do not suffer from DVT. With the growing interest in catheter-directed thrombolysis for extensive lower extremity DVT, IVCS as an anatomic risk factor for left-sided iliofemoral thrombosis is being identified with increasing frequency (25). Prior to the use of endovascular techniques, the treatment for iliofemoral DVT due to IVCS remained anticoagulation therapy and/or surgery. During the past several years, there have been several reports of favorable results in managing iliofemoral DVT due to IVCS by endovascular techniques (25, 26). O'Sullivan et al. suggest that endovascular techniques are advantageous for several reasons: (a) diagnostic venography allows for direct assessment of the degree of venous obstruction and collateralization; (b) large acute thrombus burdens can be cleared with use of catheter-directed thrombolysis, thus preserving valve function; (c) obstructive intravenous synechiae and webs (spurs) can be disrupted by means of angioplasty and stent placement; and (d) the integrity of the compressed iliac vein can be restored without apparent long-term harm to the overriding artery, with acceptable results (25).
The term "hypercoagulable state" is generally used to denote any conditions in which the normal balance between clotting and anticlotting mechanisms becomes altered in such a way that the patient is predisposed to thrombus formation. There are a number of conditions that can lead to a hypercoagulable state. Proteins C and S deficiencies are frequently described as causes of the hypercoagulable states (11-19). Proteins C and S are two of the vitamin K-dependent proteins. Activated protein C (protein Ca) inactivates factors Va and VIIIa. Protein C is activated to protein Ca 20,000 times faster than by thrombin alone through the interaction of thrombomodulin and thrombin on the endothelial cell surface (27). In addition, protein C proteolytically inactivates the inhibitor to tissue plasminogen activator, thus increasing the natural fibrinolytic activity of plasma. Protein S is a cofactor for protein C. The activity of protein Ca is increased several orders of magnitude by its nonenzymatic cofactor protein S. Proteins C and S deficiencies may be seen in both congenital and acquired forms. They are inherited in an autosomal dominant manner. In the congenital conditions, those homozygous for protein C deficiency usually die in infancy, while those heterozygous have antigenic protein C levels less than 60% of normal and present with recurrent venous thrombosis. Thrombotic events are often precipitated by another factor, such as trauma, surgery, or childbirth. Although the only established treatment for patients with thrombotic events is heparin therapy followed by life-long warfarin therapy, catheter-directed thrombolysis for acute iliofemoral DVT may be safe and effective in patients with protein C and/or S deficiency (9). Furthermore, in patients undergoing coronary thrombolysis, Gruber et al. showed an 11-fold increase in protein Ca during thrombolysis (28). They concluded that thrombolytic therapy generates at least two potent antithrombotic factors in the circulation, namely the fibrinolytic enzyme, plasmin, and the anticoagulation enzyme, protein Ca. Therefore, we speculate that endogenous protein Ca generated during thrombolysis has more potent antithrombotic effects in patients with DVT and protein C and/or S deficiency. Protein Ca may help prevent rethrombosis during or after thrombolysis.
In this small study, 4 with protein C and/or S deficiency and 2 without protein C and/or S deficiency showed IVCS after thrombolysis. Further intervention consisted of angioplasty and stent placement was performed successfully in all treated limbs without complications or clinically detectable pulmonary emboli. During catheter-directed thrombolysis, major bleeding complications are primarily related to the catheter insertion site (4). Cautious needle access under the ultrasound guidance is mandatory to avoid inadvertent puncture of adjacent vessels such as the popliteal artery. A theoretical consideration with catheter-directed thrombolysis is dislodgement of significant thrombotic material, leading to a pulmonary embolus. There is a relatively high incidence of asymptomatic pulmonary emboli in iliac venous thrombosis, as shown by scintigraphy (29). Although the true prevalence of pulmonary emboli is unknown in our study, there were no clinically significant cases of pulmonary emboli resulting in increasing dyspnea or arterial oxygen desaturation. We did not perform scintigraphy and insert caval filters.
In summary, the presence of protein C and/or S deficiency may influence clinical management. In addition to younger patients, other patients who presented with acute, massive iliofemoral DVT not precipitated by other risk factors might benefit from hypercoagulability testing. If there are no contraindications, patients with DVT associated with this hypercoagulability should be treated with full anticoagulation indefinitely. Considering that compression of the left common iliac vein by the right common iliac artery is a common normal finding during iliocaval venography, protein C and/or S deficiency may be an important predisposing factor for acute iliofemoral DVT secondary to IVCS. Also, endovascular techniques for the treatment of these patients require further evaluation with full clinical study and long-term follow up, and this presentation shows only the initial experience of this therapeutic modality as a safe and effective method for treating acute iliofemoral DVT with IVCS and protein C and/or S deficiency.
Figures and Tables
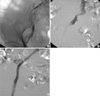
Fig. 1
A 75-yr-old female patient presents with a 10-day history of left leg pain and severe swelling extending up to the groin. Doppler ultrasound reveals acute thrombosis extending into the left common iliac vein. (A) In the prone position, ascending subtraction venogram demonstrates an acute thrombus extending into the common iliac vein. (B) After 47 hr of thrombolytic therapy, the vast majority of the thrombus has been disappeared; the underlying left common iliac vein stenosis becomes apparent. (C) After angioplasty and stent placement, final subtraction venogram shows complete resolution of iliac vein flow and no residual filling defect.
References
1. Strandness DE Jr, Langlois Y, Cramer M, Randlett A, Thiele BL. Long-term sequelae of acute venous thrombosis. JAMA. 1983. 250:1289–1292.

2. O'Donnell TF Jr, Browse NL, Burnand KG, Thomas ML. The socioeconomic effects of an iliofemoral venous thrombosis. J Surg Res. 1977. 22:483–488.
3. Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996. 125:1–7.

4. Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a National Multicenter Registry. Radiology. 1999. 211:39–49.

5. Bjarnason H, Kruse JR, Asinger DA, Nazarian GK, Dietz CA Jr, Caldwell MD, Key NS, Hirsch AT, Hunter DW. Iliofemoral deep venous thrombosis: safety and efficacy outcome during 5 years of catheter-directed thrombolytic therapy. J Vasc Interv Radiol. 1997. 8:405–418.

6. Semba CP, Dake MD. Iliofemoral deep venous thrombosis: aggressive therapy with catheter-directed thrombolysis. Radiology. 1994. 191:487–494.

8. Semba CP, Dake MD. Catheter-directed thrombolysis for iliofemoral venous thrombosis. Semin Vasc Surg. 1996. 9:26–33.
9. Cho YP, Jang HJ, Lee DH, Ahn J, Han MS, Kim JS, Kim YH, Lee SG. Deep venous thrombosis associated with protein C and/or S deficiency: management with catheter-directed thrombolysis. Br J Radiol. 2003. 76:380–384.

10. May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957. 8:419–427.

11. Cho YP, Lee DH, Jang HJ, Kim JS, Han MS, Lee SG. Peripheral arterial insufficiency associated with protein C deficiency. Br J Radiol. 2002. 75:843–846.

12. Eldrup-Jorgensen J, Flanigan DP, Brace L, Sawchuk AP, Mulder SG, Anderson CP, Schuler JJ, Meyer JR, Durham JR, Schwarcz TH. Hypercoagulable states and lower limb ischemia in young adults. J Vasc Surg. 1989. 9:334–341.

13. Lane DA, Mannucci PM, Bauer KA, Bertina RM, Bochkov NP, Boulyjenkov V, Chandy M, Dahlback B, Ginter EK, Miletich JP, Rosendaal FR, Seligsohn U. Inherited thrombophilia: Part 1. Thromb Haemost. 1996. 76:651–662.

14. Lane DA, Mannucci PM, Bauer KA, Bertina RM, Bochkov NP, Boulyjenkov V, Chandy M, Dahlback B, Ginter EK, Miletich JP, Rosendaal FR, Seligsohn U. Inherited thrombophilia: Part 2. Thromb Haemost. 1996. 76:824–834.

15. De Stefano V, Finazzi G, Mannucci PM. Inherited thrombophilia: pathogenesis, clinical syndromes, and management. Blood. 1996. 87:3531–3544.
16. Allaart CF, Poort SR, Rosendaal FR, Reitsma PH, Bertina RM, Briet E. Increased risk of venous thrombosis in carriers of hereditary protein C deficiency defect. Lancet. 1993. 341:134–138.

17. De Stefano V, Leone G, Mastrangelo S, Tripodi A, Rodeghiero F, Castaman G, Barbui T, Finazzi G, Bizzi B, Mannucci PM. Clinical manifestations and management of inherited thrombophilia: retrospective analysis and follow-up after diagnosis of 238 patients with congenital deficiency of antithrombin III, protein C, protein S. Thromb Haemost. 1994. 72:352–358.

18. Sakata T, Kario K, Katayama Y, Matsuyama T, Kato H, Miyata T. Studies on congenital protein C deficiency in Japanese: prevalence, genetic analysis, and relevance to the onset of arterial occlusive diseases. Semin Thromb Hemost. 2000. 26:11–16.

19. Sakata T, Kario K, Katayama Y, Matsuyama T, Kato H, Miyata T. Analysis of 45 episodes of arterial occlusive disease in Japanese patients with congenital protein C deficiency. Thromb Res. 1999. 94:69–78.

20. Akesson H, Brudin L, Dahlstrom JA, Eklof B, Ohlin P, Plate G. Venous function assessed during a five year period after acute iliofemoral venous thrombosis treated with anticoagulation. Eur J Vasc Surg. 1990. 4:43–48.
22. Okrent D, Messersmith R, Buckman J. Transcatheter fibrinolytic therapy and angioplasty for left iliofemoral venous thrombosis. J Vasc Interv Radiol. 1991. 2:195–197.

23. Berger A, Jaffe JW, York TN. Iliac compression syndrome treated with stent placement. J Vasc Surg. 1995. 21:510–514.

24. Cockett FB, Lea Thomas M, Negus D. Iliac vein compression: its relation to iliofemoral thrombosis and the post-thrombotic syndrome. Br Med J. 1967. 2:14–19.
25. O'Sullivan GJ, Semba CP, Bittner CA, Kee ST, Razavi MK, Sze DY, Dake MD. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2000. 11:823–836.
26. Patel NH, Stookey KR, Ketcham DB, Cragg AH. Endovascular management of acute extensive iliofemoral deep venous thrombosis caused by May-Thurner syndrome. J Vasc Interv Radiol. 2000. 11:1297–1302.

27. Clouse LH, Comp PC. The regulation of hemostasis: the protein C system. N Engl J Med. 1986. 314:1298–1304.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download