Abstract
This study was performed to assay the expression of epidermal growth factor receptor (EGFR) in non-small cell lung carcinoma (NSCLC), and to investigate the relationship between EGFR status and various clinicopathologic features of NSCLC, including angiogenesis and proliferative activity. The expression of EGFR, microvessel count (MVC) measured by CD31 monoclonal antibody, and proliferative activity using Ki-67 labeling index were immunohistochemically analyzed in formalin-fixed and paraffin-embedded tissue specimens from 65 patients with completely resected stage II-IIIA NSCLC. Pathologic and clinical records of all patients were retrospectively reviewed. EGFR was expressed in 18 (28%) of 65 NSCLC samples. More squamous tumors (35%) were EGFR-positive than other NSCLCs (23%) (p-value 0.308). There was a statistically significant correlation between EGFR expression and Ki-67 labeling index (p-value 0.042), but no correlation was observed between EGFR expression and tumor histology, stage, or MVC. There were no differences between EGFR positive and negative tumors in 5-yr disease-free survival (60% vs. 52%, p-value 0.5566) and 5-yr overall survival (53% vs. 45%, p-value 0.3382) rates. In conclusion, our findings suggest that NSCLC proliferative activity may be dependent on EGFR expression, but that EGFR expression had no significant impact on survival in curatively resected NSCLC.
Non-small cell lung cancer (NSCLC) represents both a biologically and histopathologically heterogenous group of tumors. Although complete surgical resection is the only curative therapy for NSCLC patients, the survival rate, even of patients with early stage NSCLC, is not satisfactory. This has led to a search for prognostic parameters of lung cancer from clinical data or histological examination. Recently, the potential prognostic roles of cancer cell proliferation and molecular biological alterations in oncogenes and tumor suppressor genes have been investigated in NSCLC, but none has been found to yield unambiguous results (1).
Epidermal growth factor receptor (EGFR) is a 170 kDa transmembrane glycoprotein with an intracellular domain that exhibits tyrosine kinase activity. EGFR is involved in cellular proliferation and differentiation (2, 3), and overexpression of mRNA and/or protein encoded by the EGFR gene has been observed in many types of human malignancies, including breast, gastric, colorectal, and bladder cancer, as well as in 30% to 70% of NSCLCs (4-8). Findings have suggested that assays of EGFR mRNA and/or protein may be useful for predicting the prognosis of tumors. For example, enhanced expression of EGFR has been related to poorer prognosis in breast cancer, and advanced gastric carcinomas have shown a higher level of EGFR expression than early gastric carcinomas (4, 6, 9, 10). Although some studies have reported an association between enhanced EGFR expression and poor prognosis in patients with lung cancer, the prognostic role of EGFR remains yet controversial (11-14).
In the present study, we assayed immunohistochemically EGFR expression in human NSCLC tissues, and we analyzed the relationships between EGFR expression and several prognostic variables, including angiogenesis and tumor cell proliferation. Furthermore, we investigated the prognostic role of EGFR overexpression in relation to disease-free survival and overall survival.
From February 1991 to March 1996, ninety-four patients with stage II or IIIA NSCLC underwent curative resection and received 2 courses of adjuvant MVP [mitomycin C (8 mg/m2), vinblastine (8 mg/m2), and cisplatin (60 mg/m2)] chemotherapy, followed by sequential radiotherapy (50 Gy) 3 weeks later. This protocol has shown relatively low recurrence and improved overall survival rates in patients with stage II and IIIA NSCLC (15). We obtained 65 of these resected lung tumor tissue samples from the pathology department for this study. We also reviewed the medical records and pathology data of these patients in order to determine their clinicopathologic variables, including age, sex, TNM stage, and histologic grade and types of tumors, as well as their disease free survival and overall survival.
Paraffin-embedded blocks were sectioned at about 4-µm thickness, and deparaffinized and rehydrated through a graded alcohol series. The slides were pretreated in the microwave with citrate buffer (pH 6.0) at 90℃ for 10 min to retrieve antigen, immersed in 0.3% hydrogen peroxide for 20 min to block endogenous peroxidase activity, washed, and incubated overnight at 4℃ with normal bovine serum to reduce nonspecific antibody binding. Subsequently, all tissue sections were incubated with mouse monoclonal antibody to EGFR (1:50; Novocastra Laboratories, Newcastle, U.K.) and CD31, a sensitive marker for endothelial cells (16) (JC70, 1:50; DAKO, Denmark), and with rabbit polyclonal antibody to Ki-67 protein, a marker for tumor cell proliferation (1:100; DAKO, Denmark). The slides were subsequently incubated with biotinylated goat anti-mouse antibody, and then with peroxidase-conjugated streptavidin (DAKO LSAB+ kit; Dako Corp., Carpinteria, CA, U.S.A.). Reaction products were subjected to a color reaction with 3,3-diaminobenzidine tetrahydrochloride containing 3% H2O2 in Tris buffer and were lightly counterstained with Mayer's hematoxylin.
All slides were coded and evaluated by an experienced pathologist who had no knowledge of the patients' identity or clinical status. EGFR assessment was based on cytoplasmic staining intensity was, which was scored as 0 (negative, <5% of cells stained), 1+ (weak, 5-20% of cells stained), 2+ (moderate, 20-50% of cells stained), and 3+ (strong, >50% of cells stained). Only tumors exhibiting 2+~3+ staining were considered positive for EGFR expression (17).
For microvessel count (MVC) assessment, each slide was first scanned by light microscopy at ×100 magnification to determine three "hot spots" defined as areas with the maximum number of microvessels. The slides were then examined at ×200 magnification, and three ×200 fields in each "hot spot" were evaluated by counting the number of positive cells. The highest number was recorded for statistical analysis. MVC values were expressed as mean±standard deviation (SD) (18).
Ki-67-positive cells were evaluated by nuclear staining, with the number of positive cells in 1000 tumor cells counted at high magnification (×400). The Ki-67 labeling index was calculated as positive nuclei ×100/total number of counted nuclei (%). For statistical analysis, a threshold value of 15% Ki-67 positive tumor cells were defined as separating tumors with a high proliferation rate from those with a low proliferation rate (19).
Using the SPSS 11.0 program, the relationships of EGFR expression to clinicopathologic variables, MVC, and Ki-67-based proliferative activity were analyzed by chi-square or Fisher's exact test. The significance of MVC for each clinicopathologic factor, including EGFR expression, was evaluated using Student's t test. Univariate analysis was performed to identify prognostic factors for survival by modeling Kaplan-Meier survival curves, and the difference in survival curves was compared by the log-rank test. Multivariate analysis was performed by Cox' proportional hazards regression model. Disease-free survival (DFS) was defined as the time from the date of operation to a documented recurrence or death from any other cause. Overall survival (OS) was defined as the time from the date of operation to death or last follow-up. In all statistical analyses, the difference was considered to be significant when the p-value was less than 0.05.
Sixty-five NSCLC patients (51 males and 14 females), with a median age of 58 yr (range, 34-73 yr) were studied. Of the 65 patients, 26 had squamous cell carcinoma, 29 had adenocarcinoma, 2 had bronchioloalveolar carcinoma, and 8 had adenosquamous carcinoma. Thirty-three cases were stage II and 32 were stage IIIA. The median follow-up in 33 living patients was 85.3 months (range, 18-133 months). Table 1 shows patients' characteristics and relationship with EGFR expression.
Typical immunostaining patterns for EGFR in NSCLC are shown in Fig. 1. Immunoreactivity was limited to cancer cells, and there was very little or no specific staining in the surrounding stroma. Staining for EGFR was strong in 11/65 (17%), moderate in 7/65 (11%), weak in 25/65 (38%), and negative in 22/65 (34%) tumor samples. Thus, 18/65 (28%) NSCLC tissues were positive for EGFR expression. Of the 26 squamous tumors, 9 (35%) were stained moderately or strongly, while of the 39 non-squamous tumors, 9 (23%) were positive for EGFR. Overall, more squamous tumors were EGFR-positive than other NSCLCs, but the difference was not statistically significant.
MVC, as an indicator of neovascularization in primary tumors, was measured by immunohistochemical staining of endothelial vessels with an anti-CD31 monoclonal antibody. The 65 tumors assayed had a median MVC of 95.0 per 200× field (range, 38-209). MVC was significantly associated with histologic grade but not with any other clinicopathological or biological variable analyzed (data not shown).
We found no significant association between EGFR expression and any clinicopathological parameters, including age, tumor size, pathologic stage, nodal status, histological type, and tumor angiogenesis (Table 1).
The proliferative activity of tumors was evaluated by Ki-67 expression. The mean Ki-67 labeling index was 13% (range 0-56%). We divided tumors into groups with low Ki-67 expression (Ki-67 labeling index <15%) and high Ki-67 expression (Ki-67 labeling index ≥15%). A high Ki-67 labeling index was found in 25/61 (40%) tumors. We observed a positive correlation between EGFR expression and high rate of tumor proliferation (Table 2). EGFR expression was more frequently detected in the group with high Ki-67 expression than in the group with low Ki-67 expression (61.1% vs. 38.9%, p-value 0.042).
The prognostic impact of clinicopathological variables on patient survival was evaluated by univariate analysis. Advanced tumor stage, non-squamous histologic type, high grade tumors, and high level of MVC were significantly associated with lower DFS (Table 3). EGFR expression alone, however, could not be correlated with DFS (5-yr DFS, 45% vs. 48%, p-value 0.8010) or OS (5-yr OS, 48% vs. 44%, p-value 0.4110) (Fig. 2). By multivariate analysis, only tumor stage, but not histology, histologic grade, MVC level, or EGFR expression, had a significant prognostic impact on DFS and OS (Table 4).
EGFR expression is frequently detected in many types of tumors, including the majority of NSCLC. In our study, EGFR expression was immunohistochemically evaluated in 65 formalin-fixed and paraffin-embedded human NSCLC tissues, and 18 (28%) were positive for EGFR. In previous studies by other investigators, EGFR was expressed in 30-70% of NSCLCs (4-8). In agreement with previous findings (11, 20-22), we also found that EGFR expression was more frequent in squamous cell carcinomas than in other histological types of NSCLCs (35% vs. 23%), although the difference was not statistically significant (p-value 0.308). Theoretically, high EGFR expression in squamous cell carcinomas is expected because EGF promotes the proliferation and differentiation of epidermal-like tissues. Our series of NSCLC included a relatively small population of squamous cell carcinomas (26/65, 38%), which may have affected our low EGFR expression rate.
EGFR expression is generally low in normal bronchial epithelium, whereas it is enhanced in preneoplastic and neoplastic bronchial lesions (23). Attempts to establish a relationship between EGFR expression and other clinical and pathological prognostic parameters or prognosis in primary lung cancers, however, have led to conflicting results. One report showed no correlation between EGFR expression and pathological stage, nodal status, or tumor size, although EGFR concentrations were higher in neoplastic than in normal tissues (24). A second report found that although EGFR expression was the highest in squamous cell carcinomas, it could not be correlated with other clinicopathological characteristics such as histologic grade or stage (25). In contrast, other investigators have reported significant correlations between EGFR expression and more aggressive tumor features, such as tumor stage (22) or nodal metastases (20).
In our study, no significant correlations were found between EGFR expression and several clinicopathological or biological parameters, including tumor size, nodal metastasis, histologic grade, and tumor angiogenesis. There was a correlation, however, between EGFR expression and tumor cell proliferative activity, as measured with the Ki-67 proliferation index. This index measures the proportion of cycling cells and is a potent biological marker for quantitative estimation of the growth of neoplasms (26). Binding of EGF or other ligands to EGFR is believed to trigger a series of complex signaling pathways leading to DNA synthesis and cell growth (27). Thus, our data also suggest that the proliferative activity of NSCLC may be dependent on EGFR expression.
In this study, EGFR expression had no significant impact on DFS or OS in curatively resected stage II or IIIA NSCLC. EGFR has been reported to act as a strong prognostic marker in head and neck cancers (28), genito-urinary carcinomas (29, 30) and esophageal cancers (31). In NSCLC, however, findings have been more ambiguous. Some previous studies have reported that survival rates of patients with high EGF or TGF-α levels were significantly low only in EGFR-positive tumors (32, 33). In a recent study, 5-yr survival rate of EGFR positive tumors evaluated by immunohistochemistry (IHC) was significantly lower than EGFR negative ones (34). However, several studies using specimens from larger numbers of patients suggested that EGFR expression was not associated with poor outcome (17, 20, 35). These conflicting data on the relationship between EGFR expression and survival in NSCLC may be due to heterogeneity of study populations or lack of a standardized assay for determining EGFR status. More recently, the first report of EGFR gene copy number in NSCLC using fluorescence in situ hybridization (FISH) technology showed that there was no difference in survival between the two FISH groups (low and high copy numbers of the EGFR gene) (36). However, high gene copy numbers combined with low level of EGFR protein expression by IHC had a trend toward poor prognosis. Because our study was retrospective and analyzed a relatively small number of tumors, we could not definitely determine the relationship between EGFR expression and long-term prognosis. When combined with previous results, our findings suggest that EGFR may be more important for lung tumor formation than for tumor progression. The possibility that EGFR expression plays a prognostic role in primary NSCLC should be further investigated.
Although EGFR expression may not be useful as a prognostic factor, it has potential clinical implications. The past few years have seen the rapid development of the EGFR inhibitors, and an increasing body of evidence suggests that selective inhibitors of EGFR are potential therapeutic agents for the treatment of NSCLC in adjuvant, metastatic and chemopreventive settings (37).
In conclusion, our data show no significant correlations between EGFR expression in NSCLC and other clinicopathological parameters such as tumor size, nodal metastasis, angiogenesis and prognosis. EGFR may be a useful indicator of tumor cell proliferation in stage II or IIIA NSCLC. Our results suggest the need for additional prospective studies in patients with NSCLC.
Figures and Tables
Fig. 1
Microphotograph showing EGFR immunostaining in NSCLC tissue specimens. Positive immunoreactivity is evident in the neoplastic cells (A), whereas negative immunoreactivity is shown in (B) (×100).
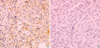
Fig. 2
Disease-free survival (DFS) curve (A) and overall survival (OS) curve (B) according to EGFR expression.

References
1. Strauss GM, Kwiatkowski DJ, Harpole DH, Lynch TJ, Skarin AT, Sugarbaker DJ. Molecular and pathologic markers in stage I non-small cell carcinoma of the lung. J Clin Oncol. 1995. 13:1265–1279.
2. Downward J, Yarden Y, Mayes E, Scrace G, Totty N, Stockwell P, Ullrich A, Schlessinger J, Waterfield MD. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984. 307:521–527.

3. Schreiber AB, Libermann TA, Lax I, Yarden Y, Schlessinger J. Biological role of epidermal growth factor-receptor clustering: investigation with monoclonal anti-receptor antibodies. J Biol Chem. 1983. 258:846–853.

4. Sainsbury JRC, Nicholson S, Angus B, Farndon JR, Malcolm AJ, Harris AL. Epidermal growth factor receptor status of histological sub-types of breast cancer. Br J Cancer. 1988. 58:458–460.

5. Hendler FJ, Ozanne BW. Human squamous cell lung cancers express increased epidermal growth factor receptors. J Clin Invest. 1984. 74:647–651.

6. Yasui W, Sumiyoshi H, Hata J, Kameda T, Ochiai A, Ito H, Tahara E. Expression of epidermal growth factor receptor in human gastric and colonic carcinomas. Cancer Res. 1988. 48:137–141.
7. Smith K, Fennelly JA, Neal DE, Hall RR, Harris AL. Characterization and quantitation of the epidermal growth factor receptor in invasive and superficial bladder tumors. Cancer Res. 1989. 49:5810–5815.
8. Veale D, Kerr N, Gibson GJ, Harris AL. Characterization of epidermal growth factor receptor in primary human non-small cell lung cancer. Cancer Res. 1989. 49:1313–1317.
9. Toi M, Nakamura T, Mukaida H, Wada T, Osaki A, Yamada H, Toge T, Niimoto M, Hattori T. Relationship between epidermal growth factor receptor status and various prognostic factors in human breast cancer. Cancer. 1990. 65:1980–1984.

10. Nicholson S, Richard J, Sainsbury C, Halcrow P, Kelly P, Angus B, Wright C, Henry J, Farndon JR, Harris AL. Epidermal growth factor receptor (EGFr); results of a 6 year follow-up study in operable breast cancer with emphasis on the node negative subgroup. Br J Cancer. 1991. 63:146–150.

11. Volm M, Drings P, Wodrich W. Prognostic significance of the expression of c-fos, c-jun and c-erbB-1 oncogene products in human squamous cell lung carcinomas. J Cancer Res Clin Oncol. 1993. 119:507–510.

12. Veale D, Kerr N, Gibson GJ, Kelly PJ, Harris AL. The relationship of quantitative epidermal growth factor receptor expression in non-small cell lung cancer to long-term survival. Br J Cancer. 1993. 68:162–165.
13. Fujino S, Enokibori T, Tezuka N, Asada Y, Inoue S, Kato H, Mori A. A comparison of epidermal growth factor receptor levels and other prognostic parameters in non-small cell lung cancer. Eur J Cancer. 1996. 32A:2070–2074.

14. Fu XL, Zhu XZ, Shi DR, Xiu LZ, Wang LJ, Zhao S, Qian H, Lu HF, Xiang YB, Jiang GL. Study of prognostic predictors for non-small cell lung cancer. Lung cancer. 1999. 23:143–152.

15. Lee SW, Choi EK, Chung WK, Shin KH, Ahn SD, Kim JH, Kim SW, Suh C, Lee JS, Kim WS, Kim DS, Kim DK, Park SI, Sohn KH. Postoperative adjuvant chemotherapy and radiotherapy for stage II and III non-small cell lung cancer (NSCLC). Lung cancer. 2002. 37:65–71.

16. Horak ER, Leek R, Klenk N, LeJeune S, Smith K, Stuart N, Greenall M, Stepniewska K, Harris AL. Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer. Lancet. 1992. 340:1120–1124.

17. Rusch V, Klimstra D, Venkatraman E, Pisters PWT, Langenfeld J, Dmitrovsky E. Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor is frequent in resectable non-small cell lung cancer but does not predict tumor progression. Clin Cancer Res. 1997. 3:515–522.
18. Dazzi C, Cariello A, Maioli P, Solaini L, Scarpi E, Rosti G, Lanzanova G, Marangolo M. Prognostic and predictive value of intratumoral microvessels density in operable non-small cell lung cancer. Lung cancer. 1999. 24:81–88.
19. Hommura F, Dosaka-Akita H, Mishina T, Nishi M, Kojima T, Hiroumi H, Ogura S, Shimizu M, Katoh H, Kawakami Y. Prognostic significance of p27KIP1 protein and Ki-67 growth fraction in non-small cell lung cancers. Clin Cancer Res. 2000. 6:4073–4081.
20. Fontanini G, Vignati S, Bigini D, Mussi A, Lucchi H, Angeletti CA, Pingitore R, Pepe S, Basolo F, Bevilacqua G. Epidermal growth factor receptor (EGFr) expression in non-small cell lung carcinomas correlates with metastatic involvement of hilar and mediastinal lymph nodes in the squamous subtype. Eur J Cancer. 1995. 31A:178–183.

21. Hendler FJ, Ozanne BW. Human squamous cell lung cancers express increased epidermal growth factor receptors. J Clin Invest. 1984. 74:647–651.

22. Veale D, Ashcroft T, Marsh C, Gibson GJ, Harris AL. Epidermal growth factor receptors in non-small cell lung cancer. Br J Cancer. 1987. 55:513–516.

23. Rusch V, Klimstra D, Linkov I, Dmitrovsky E. Aberrant expression of p53 or the epidermal growth factor receptor is frequent in early bronchial neoplasia, and coexpression precedes squamous cell carcinoma development. Cancer Res. 1995. 55:1365–1372.
24. Dittadi R, Gion M, Pagan V, Brazzale A, Del Maschio O, Bargossi A, Busetto A, Bruscagnin G. Epidermal growth factor receptor in lung malignancies. Comparison between cancer and normal tissue. Br J Cancer. 1991. 64:741–744.

25. Pfeiffer P, Nexo E, Bentzen SM, Clausen PP, Andersen K, Rose C. Enzyme-linked immunosorbent assay of epidermal growth factor receptor in lung cancer: comparisons with immunohistochemistry, clinicopathological features and prognosis. Br J Cancer. 1998. 78:96–99.

26. Viberti L, Papotti M, Abbona GC, Celano A, Filosso PL, Bussolati G. Value of Ki-67 immunostaining in preoperative biopsies of carcinomas of the lung. Hum Pathol. 1997. 28:189–192.

28. Maurizi M, Almadori G, Ferrandina G, Distefano M, Romanini ME, Cadoni G, Benedetti-Panici P, Paludetti G, Scambia G, Mancuso S. Prognostic significance of epidermal growth factor receptor in laryngeal squamous cell carcinoma. Br J Cancer. 1996. 74:1253–1257.

29. Fischer-Colbrie J, Witt A, Heinzl H, Speiser P, Czerwenka K, Sevelda P, Zeillinger R. EGFR and steroid receptors in ovarian carcinoma: comparison with prognostic parameters and outcome of patients. Anticancer Res. 1997. 17:613–619.
30. Mellon K, Wright C, Kelly P, Horne CH, Neal DE. Long-term outcome related to epidermal growth factor receptor status in bladder cancer. J Urol. 1995. 153:919–925.
31. Inada S, Koto T, Futami K, Arima S, Iwashita A. Evaluation of malignancy and the prognosis of esophageal cancer based on an immunohistochemical study (p53, E-cadherin, epidermal growth factor receptor). Surg Today. 1999. 29:493–503.

33. Tateishi M, Ishida T, Mitsudomi T, Kaneko S, Sugimachi K. Immunohistochemical evidence of autocrine growth factors in adenocarcinoma of the human lung. Cancer Res. 1990. 50:7077–7080.
34. Selvaggi G, Novello S, Torri V, Leonardo E, De Giuli P, Borasio P, Mossetti C, Ardissone F, Lausi P, Scagliotti GV. Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small cell lung cancer. Ann Oncol. 2004. 15:28–32.
35. Pfieffer P, Clausen PP, Andersen K, Rose C. Lack of prognostic significance of epidermal growth factor receptor and the oncoprotein p185HER-2 in patients with systemically untreated nonsmall cell lung cancer: an immunohistochemical study on cryosections. Br J Cancer. 1996. 74:86–91.
36. Hirsch FR, Varella-Garcia M, Bunn PA Jr, Di Maria MV, Veve R, Bremmes RM, Baron AE, Zeng C, Franklin WA. Epidermal growth factor receptor in non-small cell lung carcinioma: Correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003. 21:3798–3807.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download