Abstract
The purposes of this study were to assess the expression patterns of heat shock proteins (Hsps), after eyeball heating or cooling, and to elucidate their relationships with corneal wound healing and intraocular complications after excimer laser treatment. Experimental mice were grouped into three according to local pretreatment type: heating, cooling, and control groups. The preconditioning was to apply saline eyedrops onto the cornea prior to photoablation. Following photoablation, we evaluated corneal wound healing, corneal opacity and lens opacity. Hsp expression patterns were elucidated with Western blot and immunohistochemical staining. The heating and cooling groups recovered more rapidly, and showed less corneal and lens opacity than the control group. In the heating and cooling groups, there were more expressions of Hsps in the cornea and lens than in the control group. These results were confirmed in the Hsp 70.1 knockout mouse model. Our study showed that Hsps were induced by the heating or cooling preconditioning, and appeared to be a major factor in protecting the cornea against serious thermal damage. Induced Hsps also seemed to play an important role in rapid wound healing, and decreased corneal and lens opacity after excimer laser ablation.
Excimer laser ultraviolet radiation therapy (argon fluoride 193 nm) has been used to ablate the cornea for the correction of myopia (1). One problem with this technique, however, is the postoperative subepithelial corneal haze, which inevitably develops. Thermal loading may be a cause of side effect after excimer laser surgery of the cornea. In previous reports, the maximum temperature increase of the cornea was 7.5℃ (2, 3). Many surgeons now use corticosteroids and antimetabolites to minimize this problem. In recent studies, cooling of the cornea has been reported to be effective in reducing corneal haze induced by photorefractive keratectomy (PRK) in humans (4, 5), however, the precise mechanism of this salutary effect has not been elucidated.
Heat shock proteins (Hsps) are a group of highly conserved proteins, present in virtually all species from bacteria to human, that are rapidly induced by a variety of environmental stresses, inducing heat shock, anoxia, mechanical trauma, ethanol, glucose deprivation and heavy metals (6). Hsps can also be induced in vivo with stressors such as mild elevations in body temperature, ether anesthesia, surgery, and restraint (7). Hsps are protective proteins that aid in maintaining cell homeostasis under environmental stress (6-9). Hsps have also been called a molecular chaperone and are thought to control cell death or protect damaged cells (10, 11). The role of Hsps in interacting with other proteins and maintaining appropriate states of protein folding prior to the synthesis of other proteins, their intracellular transport or signal-specific activation, provides cells with a mechanism for enhanced production of these proteins following thermal stress when the need to inhibit inappropriate associations of partially denatured proteins will be increased (12-14). The cells that are subjected to such a stress, and which accumulate heat shock proteins, acquire a transient resistance to further episodes of oxidative stress (15). Of these proteins, the extensively studied ones are Hsp 25, 47, 70, and 90. For this reason, our study attempts to examine the expressions of Hsp 25, 47, 70, and 90 proteins which have been known important in other published studies.
We hypothesized that sublethal cellular heating or cooling induces Hsp expression, and that the induced Hsps may protect tissues from damage by excimer laser photoablation. Therefore, we examined the induction of different heat shock proteins (Hsp 25, 47, 70, and 90), after heating or cooling, and to elucidate the relationship with corneal wound healing and intraocular complications after excimer laser photoablation.
Eighty, 9-week-old, C57BL6 mice weighing between 30-40 g were used. Hsp 70.1 knockout mice (n=12) were produced in Ilchun Molecular Medicine Institute (Medical research center, Seoul National University). By crossbreeding for more than 5 generations, knockout mice share a 99% similarity in genetic background with C57BL/6 mice.
Preoperative examinations were performed under mixed anesthesia with ketamine and xylazine (0.05% ketamine: 0.03 mL and 0.023% xylazine: 0.01 mL). We performed slit lamp (Haag-Streit 900, Bern, Switzerland) biomicroscopic examinations before preconditioning to exclude any other defects such as corneal abnormality or cataract. We checked central corneal thickness with pachymetry (Sonoscan, model 4000AP, Sonomed, Lake Success, NY, U.S.A.), measured body and eye temperature of each mouse, and the environmental temperature, using a digital thermometer (Barnant 90 Type K thermocouple model No 600-2840, Barnant, Sweden).
Experimental mice were divided into three groups. In Group H, the heating group (n=22), the eyes were exposed to 45℃ saline by dripping for 40 min. This irrigation was done in the laboratory where ambient temperature was 44℃. The irrigation was performed for 40 min while corneal surface temperature of 43℃ was checked with the digital thermometer. In Group C, the cooling group (n=26), the eyes were exposed to 4℃ saline by dripping for 40 min. Group N was the control group (n=32). Corneal temperature in this group was 30.6-30.8℃, and body temperature was 32.3-32.6℃ at room temperature, so we exposed the eyes in this group to 32℃ warm saline by dripping it into them for 40 min. After preconditioning, each group was allowed to recover for 6 hr at room temperature. Our cell culture study showed that maximal expression of Hsp 70 occurred 6 hr after an inductive event, so recovery was provided for 6 hr.
We used an argon fluoride excimer laser (Mel-60, Meditec, Jena, Germany), emitting at 193 nm. A phototherapeutic keratectomy (PTK) spot mode was used to transmit the beam. An ablation rate of 1.5 µm per pulse was calculated. The repetition rate was 5 Hz. The diameter of the ablation zone was 2.0 mm (using the device with an inner diameter of 2.0 mm). The intended ablation depth through the epithelium and stroma was approximately 45 µm. Six hours after preconditioning, the pupil was dilated under anesthesia. The corneal epithelium was removed by scraping with a cottontipped stick soaked in 70% alcohol for 30 sec, and then the excimer laser was applied.
After applying the laser, we evaluated the corneal wound quantitatively every 24 hr until it healed completely, using magnified photography (MF-21 camera, Nikon, Japan, ×30). The corneal wound was stained with fluorescein and photographed postoperatively with a slit-lamp camera. The wound was surveyed using Scion image software (beta version 3b for Windows, Scioncorp, Frederick, MD, U.S.A.), and three ophthalmologists who independently recorded their results. The mean area was used for this study. After the wound had healed completely, we dilated the pupil with neosinephrine eyedrops every day, and evaluated corneal and lens opacity at 2 weeks after excimer laser treatment. The results were divided into different grade levels (Table 1, 2, Fig. 2, 3).
To identify Hsps in the cornea and lens, Western blot analysis was performed using Hsp-specific antibodies. After using the protocol described above for heating or cooling the eyes for 3, 6, 12, or 24 hr, the mice were killed, the eyeballs enucleated, and the cornea and lens were removed from the eyeball. The cornea and lens were disrupted by freezing in liquid nitrogen. Extracts were prepared by grinding in RIPA buffer (150 mM NaCl, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecylsulfate (SDS), and 50 mM Tris HCl at pH 8.0) with protease inhibitor cocktail (0.1 mM pepstatin, 0.1 mM antipapain, 0.1 mM chymostatin, 0.2 mM leupeptin, 1 mM phenylmethylsulfonyl fluoride). The solution of ground tissues was passed through a 20-gauge needle five times using a 1 mL syringe, allowed to sit at 4℃ for 15 min, and then centrifuged at 12,000 g and 4℃ for 5 min. The supernatant was used in Western blot analysis. Protein concentration was determined by the Bradford assay.
The extracts of cornea and lens (100 µg) were subjected to SDS-PAGE in 10% (w/v) acrylamide gels and the separated proteins were transferred to nitrocellulose membranes using a protein transfer system. Blots were blocked by incubating in Tris-buffered saline containing 5% (w/v) non-fat milk and 0.1% (v/v) Tween-20 (T-TBS) at 4℃ for 4 hr. They were then probed with polyclonal antibodies (primary antibody) specific for Hsp 70 (at 1/1,000 dilution) for 2 hr. Then, blots were washed and incubated with horseradish peroxidase-conjugated anti-mouse IgG (Bio-Rad, Hercules, CA, U.S.A.) and visualized using the Amersham ECLTM system (Amersham Pharmacia Biotech, Inc.). The antibodies used were mouse anti-Hsp monoclonal antibody; anti-Hsp 25, 47, 70, and 90 (StressGen Biotechnologies Corp., Victoria, British Columbia, Canada).
At the intervals of 3, 6, 12 and 24 hr after heat or cold shock, the expression and localization of Hsps were examined by immunohistochemical staining. All the enucleated mouse eyes were placed in fixative within about 24 hr. After fixation, they were cut equatorially behind the ora serrata, the anterior segment was dissected into quarters, and were paraffin-embedded. The cut-sections were deparaffinized, and treated with hydrogen peroxide to eliminate endogenous peroxidase activity. After, PBS washing sections were then treated with blocking serum, incubated for 2 hr with anti-Hsp monoclonal primary antibodies against each Hsp. After PBS washing, the specimens were incubated with secondary antibody, and treated with enzyme conjugate, washed again with PBS, treated with AEC, washed with distilled water, and then stained with hematoxylin for 1 to 3 min. The sections were rinsed, dehydrated, cleared, and mounted, then examined microscopically and photographed. The antibodies used were same with those used in the Western blot.
For statistical analysis, SPSS/PC software (version 8.0 for Windows, SPSS Inc.) was used. The statistical significance of differences among groups was evaluated in all cases using the ANOVA for the epithelial defect area, corneal opacity, and lens opacity. Differences were considered significant if p<0.05.
The central corneal thickness of mice was 329 to 331 µm. When, ambient temperature was 27℃ by using the digital thermometer, the body temperature of mice ranged from 32.3-32.6℃ at the skin, 36.9-37.0℃ in the external auditory canal, 30.6-30.8℃ at the cornea, and 32.3-32.6℃ at the eyelid. After warm saline was placed into the eye using an eyedropper the corneal temperature was 43℃ and the eyelid temperature 38.8℃. After cold saline irrigation, the corneal temperature was 6℃ and eyelid temperature was 20.6℃. When Application of excimer laser on a plastic table raised the local temperature up to 59℃. After application to the cornea, however, the corneal temperature was changed up to 38.8℃.
After 72 to 120 hr, all laser wounds healed completely. Group H and Group C showed more rapid wound healing than Group N (Fig. 1). In the Hsp 70.1 knockout mice (n=12), corneal wounds healed slower than those in other 3 groups. After 96 to 144 hr, all laser wounds healed completely. We analyzed the three groups based on the rate of healing after 72 hr (Table 3). Twenty eyes in Group H were completely healed (66.7%), 17 in Group C (50%) and 14 in Group N (43.7%). The remaining mean epithelial defect area after PTK in Group N of 129.1 mm2 was much larger than 41.6 mm2 in Group H (p<0.05) (Table 4).
Hazy cornea after PTK, observed in the early postoperative period, is usually considered to be corneal edema. Corneal opacities at 2 weeks after laser treatment were divided into different grade levels (Fig. 2). Eleven eyes (50%) fell into grade 0.5 in Group H, and 16 eyes (50%) fell into grade 2 in Group N (Table 5). The difference in the grades of Groups H and N was statistically significant (p=0.01). In the Hsp 70.1 knockout mice, grade 1 corneal opacity was present in 1 eye, grade 2 in 7 eyes, and grade 3 in 4 eyes.
Lens opacity was observed 1 day after laser treatment, which started at the Y suture line and progressed to the mature form of cataract (Fig. 3). Lens opacity development after PTK was more prevalent in Group N than in Groups H and C (Table 6). Three eyes in Group H, 1 eye in Group C, and 1 eye in Group N were grade 0. One eye in Group H and 4 eyes in Group N were grade 4. The difference in the lens opacity grade between Groups H and N was statistically significant (p=0.026). In the Hsp 70.1 knockout mice, grade 2 lens opacity was present in 2 eyes, grade 3 in 6 eyes, and grade 4 in 4 eyes. Other two intraocular complications were found. One is hyphema and the other is severe corneal neovascularization. These two complicated cases were excluded.
The maximal induction of Hsp 47 occurred at 3 hr after preconditioning in Group H and 24 hr in Group C as observed by Western blot (Fig. 4, Table 7). Western blot studies in the control, Group N, and an untreated normal group, showed no differences between them. In the corneas of Group N there was no Hsp 47 induction. Small amounts of Hsp 70 were seen in Group N control corneas, whereas the maximum amount of Hsp 70 was seen 3 hr after heat induction in Group H, and 24 hr after cold treatment in Group C. We did not observe any changes in Hsp 27 or 90 in the corneas or lenses.
We confirmed the increased expression of Hsp in the cornea and lens after heating and cooling (Fig. 4). In the cornea, densitometry showed Hsp 70 peaking to 158 in Group C and to 287 in Group H at 6 hr after preconditioning. Hsp 47 peaked to 138 in Group C and to 147 in Group H at 3 hr after preconditioning. Hsp 25 peaked to 741 in Group C and to 620 in Group H at 6 hr after preconditioning. After these peaks, the Hsp levels decreased gradually. Hsp 90 did not change notably in Group C. In the lens, Hsp 25 peaked to 480 in Group C at 3 hr and to 387 in Group H at 6 hr after preconditioning. Other Hsps were not seen in the lens.
In the histologic findings, maximal expression after preconditioning occurred at the same times found in the Western blotting study. Seen with immunohistochemical staining, the degrees of Hsp 25, 47, and 70 expressions overall showed slight differences, but the expression patterns were similar (Fig. 5, Table 7). Although the overall expression pattern of Hsp 25 was similar to that of Hsp 47, the increased level after preconditioning was not as large as that of Hsp 47. Hsp 47 is normally expressed weakly in the limbus keratocytes and the entire endothelium, but was expressed strongly throughout all layers of the cornea 6 hr after heating and 12 hr after cooling, especially in the corneal basal cell layer and endothelium. In control Group N, Hsp 47 was not expressed in the lens epithelium. But it was expressed strongly in both preconditioned Groups H and C. In Group N, Hsp 70 was expressed in keratocytes of the limbus and in the entire endothelium, but was not expressed in the central cornea. In Groups H and C, the maximal expression of Hsp 70 was 12 hr after preconditioning. At this time, the expression was not only in the endothelium but also in basal cells and fibroblasts in the limbus and central cornea. Overall, Hsp 25, 47, and 70 were expressed relatively early at 3 and 6 hr after heating, and were expressed at 12 and 24 hr after cooling. These results were similar to those of Western blotting.
Exposure of cells to sublethal stresses such as heat or cold have been shown to lead to the synthesis of several families of heat shock proteins (Hsps) at elevated levels. Hsps are classified according to their molecular masses (Hsp 27-27 kDa, Hsp 47-47 kDa, Hsp 70-70 kDa, Hsp 90-90 kDa, etc.). Mouse Hsp 25 (Hsp 27 in humans), a member of the low molecular weight family of Hsps, is expressed constitutively and appears to have important roles in conferring protection against various cytotoxic stimuli (16). Hsp 47 has been reported to be associated with the metabolism or processing of procollagen as a molecular chaperone specific to collagen (17), and is believed to play an important role in development because collagen biosynthesis is one of the major molecular events driving embryogenesis. Conveniently enough, various forms of the Hsp 70 family can be well distinguished immunologically. Both constitutive and inducible forms exist (19). The constitutive form is expressed in unstressed cells, and the inducible form is expressed only in stressed cells. Synthesis of both forms increases after hyperthermia (20). The major heat shock protein Hsp 70 can be synthesized in a wide variety of cells such as rat islet cells, cardiomyocytes or cultured hepatocytes in response to heat or cold treatment, and it is known that the degree of Hsp 70 induction varies according to the recovery time after the thermal stress (20-22). Polla demonstrated that Hsp may have a direct antioxidant effect and that Hsp 70 may play a particularly critical role in producing thermotolerance (15). Hsp 90 is one of the most abundant proteins in mammalian cells and appears to have a role in regulating the activity of intracellular proteins such as steroid hormone receptors and protein kinase (23). Although there have been many past studies on Hsp, our study is the first to investigate Hsp induction from the perspective of using it as a preconditioning treatment for corneal tissue in an in vivo system.
Induction of Hsps has been shown to protect against the cytotoxic effects of oxidative stresses and confer thermal resistance, thereby allowing cells to withstand higher temperatures, even normally lethal ones (24, 25). In mammalian tissue culture cells, the heat shock response is typically induced by elevating the ambient temperature from 37℃ to above 41℃, with most studies utilizing supra-physiologic temperatures of 42-45℃ (2, 23, 26-29, 38). An interval that allowed for 6 hours of recovery after heat treatment was very effective in protecting DNA from damage due to chemical stresses. In this study, we maintained the corneal superficial temperature at 43℃ for 40 min, and then the mice were allowed to have a 6-hr recovery period at room temperature. Currie and Tanguay (27) demonstrated that certain sublethal stresses could lead to the expression of specific proteins, which appear to either offer or be associated with tolerance and resistance to subsequent exposure to stress.
In this study, the heating and cooling groups, which overexpressed Hsps as a result of preconditioning, showed more rapid wound healing and less corneal opacity than the control group. Lens opacity was also obviously less than in the control group. This may be due to Hsp 25 and α-crystallin. Another reason may be that the preconditioning of the shallow anterior chamber of the mouse may have allowed significant expression of Hsp in the lens epithelium, and this in turn may have produced thermo-tolerance to the eximer laser, reducing its deleterious effects. Further research into this possibility is required. The comparison between the heating group and the control group was statistically significant (p<0.05). This finding may be evidence that over-expressed Hsps play an important role in the enhancement of thermo-tolerance in the cornea and lens. This is consistent with the finding that protection against light-induced degeneration of rat retinal photoreceptors is conferred by prior whole-body hyperthermia, which induces retinal Hsp 70 (7). Yamaguchi et al. (30) reported that the corneal epithelium contains low levels of Hsp 70 immuno-reactivity under normal conditions and that those levels were elevated after hyperthermia. After the preconditioned groups recovered in our study, stress was reapplied when the Hsps were maximally induced, and subsequent rapid wound healing, and decreased corneal and lens opacities were noted. To investigate the role of Hsps more in depth, Hsp 70, the most widely known and specific among the Hsps, was evaluated in a Hsp 70-deficient knockout mouse model. Of the many subgroups of the inducible form of Hsp 70; Hsp 70.1 and Hsp 70.3, a knockout mouse study of Hsp 70.1 was conducted. Although the number of knockout mice was small, they showed a trend for slower wound healing, and more corneal and lens opacity than the other mice.
Our results show that some Hsps are expressed constitutively in the cornea and lens. The tissues facing the anterior chamber of the eye are exposed constantly to aqueous humor containing reactive oxygen products generated by light-catalyzed reactions. Of these, hydrogen peroxide is thought to originate from a light-catalyzed oxidation of ascorbic acid (31). In general, hydrogen peroxide and ascorbic acid are reactants in the mixed function oxidation process that can generate a number of free radicals (32). These free radicals may cause increased oxidation in tissues of the anterior eye segment and finally lead to oxidative damage. Such constitutive expression in the cornea and lens might be due to continual oxidative stress.
As for pathological findings; at 12 hr after preconditioning-the time of increased Hsp expression after heating and cooling, inflammation of tissues could be seen, suggesting that this weak inflammation would prepare the tissues for further inflammation and increase their resistance. In our immunohistochemical study to localize Hsps, we observed weak immunostaining for Hsp 70 in the basal cells and endothelium of the control corneas, suggesting constitutive expression of Hsp 70 in the corneal epithelium. After heat shock, however, Western blot revealed a large amount of Hsp 70 induction. The density of the immunoreaction product in the corneal epithelium became greater, especially at the limbus, reflecting an increased accumulation of Hsp 70. Hsp 47 was not found in the central cornea and lens of the control group. In contrast to the control, Hsp 47 immunoreactivity in the heat and cold shock groups was found in basal cells, fibroblasts of the cornea, and endothelium and epithelium of the lens.
Although most molecular chaperones characterized to date have been shown to bind non-selectively to denatured or immature proteins, Hsp 47 is a 'specific chaperone' for collagen in terms of its binding specificity (17, 33-37). Hsp 47 has been reported to bind to type I-V collagen in vitro (38), and its expression has been shown to be correlated with both types I and III collagen in vivo (39). In addition, Hsp 47 is reported to be associated as a molecular chaperone with α1-collagen expression (36). α1 (IV)-collagen mRNA has been detected in the lens, hyaloid vessels, and retina during ocular development (40). The functional role of Hsp 47 is not clear, but in light of these facts, Hsp 47 may play an important role in reducing corneal and lens opacity. Our evidence showed that Hsp 47 induced in the cornea and lens may confer increased stability and resistance against excimer laser damage, thus it would be of further interest to study its role in the eye.
In order to clarify the role of Hsp, Hsp 70.1 was selected and for the past year, experiments were conducted on a knockout mouse model to study wound healing and opacity of the cornea after an excimer laser procedure. Since past studies have predominantly dealt with Hsp 70, these experiments were conducted to confirm our previous conclusions, using the Hsp 70.1 knockout mouse. We feel the conclusions of our studies were subsequently confirmed in the Hsp 70.1 knockout model.
In conclusion, sublethal preconditioning is effective in reducing corneal and lens opacity and increasing the wound-healing rate after excimer laser treatment. In the preconditioning groups, there were more expressions of Hsps in the cornea and lens than in the control group, although small amounts of Hsp 70 are normally found in the endothelium. Together, basal expression and stress-induced induction of heat shock proteins could play an important role in cellular protection against physiologic or environmental stress.
The fact that Hsps normally exist in the limbus and endothelium suggests that these Hsps play a very important role of defense in vital cells. This role was confirmed in the knockout mouse case. Although the knockout study examined only Hsp 70.1, one of many Hsps, delayed wound healing, and increased corneal and lens opacity were noted, compared to the control group. In addition, induced Hsp 47 in the lens epithelium showed evidence of protection against cataract formation. In light of these facts, the increased levels of Hsp 70 and Hsp 47 appeared to be major factors in the protection of the cornea and lens against lethal thermal damage, as well as played an important role in rapid wound healing, and decreased corneal and lens opacity after excimer laser ablation.
This study demonstrated that thermal preconditioning has a potential to induce larger amounts of Hsps and to enhance thermo-tolerance against excimer laser photoablation. Further experimental studies are required, however, but we think that the induction of heat shock protein by heat shock and cold shock before excimer laser ablation can be used as a new therapeutic modality.
Figures and Tables
Fig. 1
Corneal wound after laser treatment by time interval. At 72 hr after laser treatment, in Group H (A-C), the wounds are completely healed with no visible fluorescein-stained area. In Group C (D-F), the wounds are largely healed, but small wounds remain. In Group N (G-I), and the knockout mice (J-L), large, incompletely healed wounds remain.
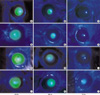
Fig. 2
Corneal opacity (two weeks after laser treatment). The pictures above are representative cases of Grade 0.5 (A), Grade 1 (B), and Grade.

Fig. 3
Lens opacity. Biomicroscopic pictures of mouse lenses, two weeks after excimer laser application. The pictures above are representative cases of Grade 0 (A), Grade 1 (B), Grade 2 (C), Grade 3 (D), Grade 4 (E), and the knockout mice (Grade 4 (F)).

Fig. 4
Western blot. In heating group (A), Hsp 70 is seen normally but increased progressively by 3 hr after heating and peak at 12 hr and then decreased until 24 hr. And Hsp 47 is not visible in normal cornea. Three hours after heat treatment, however, Hsp 47 is at the peak level and then decreased progressively. In cooling group (B), Hsp 70 is seen normally but increased progressively at 3 hr after cooling and maintained this level at 24 hr. And Hsp 47 is not visible at normal cornea, however, 12 hr after cooling, small amount of Hsp 47 is seen and reached the peak level at 24 hr. The resulting values (C, D) are obtained by densitometry.
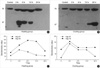
Fig. 5
Immunohistochemical localization of Hsps in the cornea and lens after excimer laser photoablation. Hsp 25 in the control group shows weak expression in keratocytes (A) and no expression in lens epithelium (data not shown), but shows increased expression in keratocytes (arrow) of the limbus (B) and the lens epithelium (arrow) at 12 hr after heating (C). Although Hsp 45 is normally expressed faintly in fibroblasts of the limbus, it was not expressed in the central cornea and lens epithelium of the control group (data not shown). Hsp 45 is expressed strongly in both the central cornea and limbus 24 hr after cooling (D) and 6 hr after heating (E). Hsp 45 is expressed strongly in both the central cornea and limbus 24 hr after cooling (D) and 6 hr after heating (E), however, is also expressed in the lens epithelium (arrow) (F). Hsp70 is normally expressed in keratocytes of the limbus and endothelium (data not shown), and increased in overall level of expression 12 hr after heating (G) and cooling (H).

Table 3
Corneal wound healing after excimer laser treatment in different groups. Analysis based on complete wound healing by 72 hr

Table 4
Comparison of the remaining areas of corneal epithelial wound at 72 hr after laser treatment (mean (mm2)±S.D.)

ACKNOWLEDGEMENT
This study was supported by a grant of the Korean Health 21 D&D Project, Ministry of Health and Welfare, Republic of Korea (Pjl-pg3-20500-0041).
References
1. Marshall J, Trokel SL, Rothery S, Krueger RR. Long-term healing of the central cornea after photorefractive keratectomy using an excimer laser. Ophthalmology. 1988. 95:1411–1421.

2. Langenbucher A, Seitz B, Kus MM, Nauman GO. Thermal effects in excimer laser trephination of the cornea. Graefes Arch for Clin Exp Ophthalmol. 1996. 234:Suppl 1. S142–S148.

3. Bende T, Seiler T, Wollensak J. Corneal thermal gradients. Grafes Arch for Clin Exp Ophthalmol. 1988. 226:277–280.
4. Tsubota K, Toda I, Itoh S. Reduction of subepithelial haze after photorefractive keratectomy by cooling the cornea. Am J Ophthalmol. 1993. 115:820–821.

5. Park WC, Tseng SCG. Temperature cooling reduces keratocyte death in excimer laser ablated corneal and skin wounds. Invest Ophthmol Vis Sci. 1988. 39:Suppl 1. 2062.
6. Tanaka Y, Kobayashi K, Kita M, Masuda H, Kinoshita S, Nakata K, Imanishi J. Expression of 47 kDa heat shock protein (HSP47) during development of mouse cornea. Exp Eye Res. 1996. 63:383–393.

7. Barbe MF, Tytell M, Gower DJ, Welch WJ. Hyperthermia protects against light damage in the rat retina. Science. 1988. 241:1817–1820.

8. Laios E, Rebeyka IM, Prody CA. Characterization of cold-induced heat shock protein expression in neonatal rat cardiomyocytes. Mol Cell Biochem. 1997. 173:153–159.
9. Tamm ER, Russell P, Johnson DH, Piatigorsky J. Human and monkey trabecular meshwork accumulate α-crystallin in response to heat shock and oxidative stress. Invest Ophthalmol Vis Sci. 1996. 37:2402–2413.
11. Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993. 62:349–384.

12. Lindquist S, Craig EA. The heat shock proteins. Annu Rev Genet. 1988. 22:631–677.
13. Morimoto RI, Tissieres A, Georgopoulos C. Morimoto RI, Tissieres A, Georgopou;os C, editors. Progress and perspectives on the biology of heat shock proteins and molecular chaperones. Biology of heat shock proteins and molecular chaperones. 1994. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press;1–30.
16. Jakob U, Buchner J. Assisting spontaneity: the role of hsp90 and small hsp as molecular chaperones. Trends Biochem Sci. 1994. 19:205–211.
17. Hirayoshi K, Kudo H, Tacheshi H, Nakai A, Iwamatsu A, Yamada KM, Nagata K. HSP 47: a tissue-specific transformation-sensitive, collagen-binding heat shock protein of chicken embryo fibroblasts. Mol Cell Biol. 1991. 11:4036–4044.
18. Brown CR, Martin RL, Hansen WJ, Beckmann RP, Welch WJ. The constitutive and stress inducible forms of hsp 70 exhibit functional similarities and interact with one another in an ATP-dependent fashion. J Cell Biol. 1993. 120:1101–1112.

19. Muchowski PJ, Valdez MM, Clark JI. AlphaB-crystallin selectively targets intermediate filament proteins during thermal stress. Invest Ophthalmol Vis Sci. 1999. 40:951–958.
20. Kim YM, de Vera ME, Watkins SC, Billar TR. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor alpha induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997. 272:1402–1411.
21. Bellmann K, Wenz A, Radons J, Burkart V, Kleemann R, Kolb H. Heat shock induces resistance in rat pancreatic islet cells against nitric oxide, oxygen radicals and streptozotocin toxicity in vitro. J Clin Invest. 1995. 95:2840–2845.

22. Laios E, Rebeyka M, Prody CA. Characterization of cold-induced heat shock protein expression in neonatal rat cardiomyocytes. Mol Cell Biochem. 1997. 173:153–159.
23. Koroshetz WJ, Bonventre JV. Heat shock response in the central nervous system. Experientia. 1994. 50:1085–1091.

24. Polla BS, Bonventre JV, Krane SM. 1,25-dihydroxyvitamin D3 increases the toxicity of hydrogen peroxide in the human monocytic line U937: Role of calcium and heat shock. J Cell Biol. 1988. 107:373–380.
25. Spitz DR, Dewey WC, Li GC. Hydrogen peroxide or heat shock induces resistance to hydrogen peroxide in Chinese hamster fibroblast. J Cell Physiol. 1987. 131:364–373.
26. Li GC, Li L, Liu YK, Mak JY, Chen LL, Lee WM. Thermal response of rat fibroblasts transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci USA. 1991. 88:1681–1685.
27. Currie RW, Tanguay RM. Analysis of RNA for transcripts for catalase and SP71 in rat hearts after in vivo hyperthermia. Biochem Cell Biol. 1991. 69:375–382.

28. Suzuki K, Sawa Y, Kanada Y, Ichikawa H, Shirakura R, Masuda H. In vivo gene transfection with heat shock protein 70 enhances myocardial tolerance to ischemia-reperfusion injury in rat. J Clin Invest. 1997. 99:1645–1650.

29. Yellon DM, Latchman DS. Stress proteins and myocardial protection. J Mol Cell Cardiol. 1992. 24:113–124.
30. Yamaguchi K, Barbe MF, Brown JR, Tytell M. Induction of stress (heat shock) protein 70 and its mRNA in rat corneal epithelium by hyperthermia. Curr Eye Res. 1990. 9:913–918.

31. Pirie A. A light-catalysed reaction in the aqueous humor of the eye. Nature. 1965. 205:500–501.
32. Stadtman ER. Oxidations of proteins by mixed function oxidation systems.: Implications in protein turnover, aging and neutrophil function. Trends Biochem Sci. 1986. 11:11–12.
33. Nagata K, Saga S, Yamada KM. A major collagen-binding protein of chick embryo fibroblast is a novel heat shock protein. J Cell Biol. 1986. 103:223–229.
34. Nagata K, Yamada KM. Phosphorylation and transformation sensitivity of a major collagen binding protein of fibroblasts. J Biol Chem. 1986. 261:7531–7536.
35. Saga S, Nakata K, Chen WT, Yamada KM. pH-dependent function, purification, and intracellular location of a major collagen-binding glycoprotein. J Cell Biol. 1987. 105:517–527.

36. Nakai A, Hirayoshi K, Nagata K. Transformation of BALB/3T3 cells by Simian virus 40 causes a decreased synthesis of a collagen-binding heatshock protein (HSP 47). J Biol Chem. 1990. 265:992–999.
37. Takechi H, Hirayoshi K, Nakai A, Kudo H, Saga S, Nakata K. Molecular cloning of a mouse 47-kDa heat shock protein (HSP47), a collagen binding stress protein, and its expression during the differentiation of F9 teratocarcinoma cells. Eur J Biochem. 1992. 206:323–329.
38. Natsume T, Koide T, Yokota S, Hirayoshi K, Nagata K. Interactions between collagen-binding stress protein HSP 47 and collagen. Analysis of kinetic parameters by surface plasmon resonance biosensor. J Biol Chem. 1994. 269:31224–31228.
39. Masuda H, Fukumoto M, Hirayoshi K, Nagata K. Coexpression of the collagen-binding stress protein HSP47 gene and the α1(I) and α1(III) collagen genes in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1994. 94:2481–2488.
40. Sarthy V. Collagen IV mRNA expression during development of the mouse retina: an in situ hybridization study. Invest Ophthalmol Vis Sci. 1993. 34:145–152.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download