Abstract
Arterial thrombosis is relatively rare compared with venous thrombosis in nephrotic syndrome. However, the assessment of its pathogenesis and risk factors in individual patient with nephrotic syndrome is necessary to allow appropriate prophylactic management because it is a potentially serious problem. Hereby, with review of the literature, we report a case of a 53 yr-old man with cerebral infarction associated with nephrotic syndrome due to focal segmental glomerulosclerosis during the course of treatments with diuretics and steroid. It reveals that the hypercoagulable state in nephrotic syndrome can be associated with cerebral infarction in adults. Prophylactic anticoagulants can be considered to reduce the risk of serious cerebral infarction in nephrotic patients with risk factors such as severe hypoalbuminemia and on diuretics or steroid treatment, even in young patients regardless of types of underlying glomerular diseases.
Since Pierre Rayer first reported renal vein thrombosis associated with nephrotic syndrome in 1837, thrombosis is known to be one of the main complications of nephrotic syndrome with the renal vein being the most frequent site (1-5). Also since Fishberg first drew general attention to the arterial thrombi in 1954, both venous and arterial thrombosis have been observed in nephrotic patients. Although arterial thrombosis has rarely been reported and is mainly observed in children, it is a potentially serious problem in nephrotic patients. Therefore awareness of its pathogenesis and assessment of the risk factors for arterial thrombosis are required to allow appropriate prophylactic management. Up to now, however, there are no reliable risk factors to suggest anticoagulation therapy in patients with nephrotic syndrome and prophylactic anticoagulants remains controversial.
We report a case of cerebral infarction in a 53-yr-old man with nephrotic syndrome with a review of cerebral infarction in adults associated with nephrotic syndrome in English and Korean literatures to identify predictive risk factors and to reveal potential benefits of prophylactic anticoagulants.
A 53-yr-old man was hospitalized in April 2002 because of a sudden motor weakness in his left extremities, dysarthria, right eye visual disturbance, and headache. A diagnosis of nephrotic syndrome had been made elsewhere in 3 weeks before admission, based on clinical manifestation, blood, and urinary abnormalities and focal segmental glomerulosclerosis was confirmed by percutaneous renal biopsy. Also he had been diagnosed with alcohol-associated liver cirrhosis (Child-Pugh Classification A) from abdominal ultrasonography and healthy HBV carrier in 7 months before admission. After diagnosis of focal segmental glomerulosclerosis, he had been treated with diuretics, angiotensin converting enzyme (ACE) inhibitor, prednisolone 1 mg/kg/day, and lamivudine with a dosage of 100 mg/day for the prevention of flare up of viral hepatitis. He had no history of hypertension or diabetes. He had been a 30-pack year smoker and a heavy alcoholic, but quitted smoking and alcohol several months before. On admission, his general appearance revealed that he was acutely ill with bilateral pedal edema. His blood pressure was 120/80 mmHg, pulse rate 120/min, respiratory rate 24/min, and body temperature 37℃. Cardic examination showed normal findings. He had no evidence of systemic thromboembolism, deep vein thrombosis, or cervical bruit. Neurological examination revealed drowsy mentality with a left-side motor weakness, and decreased sensory perception on the left side. He was also exhibited dysarthria and right eye visual disturbance. However, the high cortical function was normal.
The initial MR and MR angiography of the brain showed a recent infarction in the right middle cerebral artery territory and the head of the caudate nucleus, no blood flow signal intensity in the right internal carotid artery (Fig. 1, 2).
Laboratory data showed leukocytosis (2.49×103/µL) and hematocrit of 31.9% with hemoglobin of 10.9 g/dL, and platelet of 230×103/µL. The serum sodium level was 136 mEq/L, potassium 4.1 mEq/L, blood urea nitrogen 33 mg/L, serum creatinine 1.0 mg/L, and total serum protein 3.3 g/dL with albumin 1.2 g/dL. Urinalysis revealed 4+ albuminuria and 24-hr urine protein over 7.8 g/day. The serum cholesterol level was elevated at 539 mg/dL. The liver function tests, prothrombin time, and activated partial thromboplastin time were normal. The fibrinogen concentration and antithrombin III level were normal. The concentration of free protein S was 35.0% (normal 70-140%). Plasminogen and platelet aggregation test were not performed. The negative HBe Ag, undetectable HBV DNA, and HBs Ag seroconversion after lamivudine therapy were demonstrated. The C3 and C4 were in normal ranges. Anticardiolipin (IgM) was 4.9 MPL unit/mL (normal <12 MPL unit/mL). After the admission, supportive care for the cerebral infarction was initiated and he received anticoagulation therapy later. Hospital course was uneventful with a mild degree of residual left-side motor weakness at discharge. Two months after discharge, remission of nephrotic syndrome occurred on medication with steroid, angiotensin II receptor blocking agent. At present, 1 yr after discharge, serum albumin level is 4.6 g/dL with serum cholesterol 158 mg/dL and negative albuminuria on tapered dose of prednisolone 7.5 mg every other day (QOD).
Thromboembolic complication has been well known as a clinically important sequelae and its risk is higher in patients with nephrotic syndrome than in any other condition encountered in internal medicine (1). Although its accurate pathogenesis remains unclear, hypercoagulability is regarded as a major factor for thrombosis in nephrotic patients (1-8).
A disequilibrium between the clotting activator system and the inhibitor system may be important in the hypercoagulability of nephrotic syndrome.
The study of Kanfer et al. (5) and Kendall et al. (2) demonstrated a reduction in the concentration of low molecular weight coagulation factors (factor IX, XI), probably resulting from increased urinary loss. This is accompanied by an elevated level of high molecular procoagulatory cofactors (factor V, VII, VIII, and X) and the fibrinogen level. It is inversely correlated with the depression of serum albumin.
Regulatory proteins of the coagulation cascade are also subject to alterations in nephrotic syndrome. Kauffmann et al. reported low plasma antithrombin III levels below 70% of normal in eight of the nine patients studied and a significant negative correlation between the antithrombin III concentraion and the urinary protein excretion. This depletion of antithrombin III has been confirmed and shown to be the result of urinary losses of this low-molecular weight protein (3). However, high concentrations of antithrombin III have also been reported (2) as well as high and low levels in other series. Meanwhile, Andrassy et al. noted that only patients with a serum albumin level below 2.0 g/dL showed low level of antithrombin III, and that thromboembolic complications occurred in some patients with normal antithrombin III levels (1). Also antithrombin III concentrations increase in response to administration of steroids.
The fibrinolytic system is disturbed in nephrotic syndrome. Plasminogen levels have been generally reported as low because of urinary losses of protein, while α2-antiplasmin and α2-macroglobulin are increased (5).
Vigano-D'Angelo et al. observed low level of free protein S in patients with nephrotic syndrome as a result of urinary loss of low molecular weight free protein S. This precludes formation of activated protein C-protein S complexes required for inhibition of thrombin activity resulting in a hypercoagulable state (9).
In patients with nephrotic syndrome, increased adenosine diphosphate (ADP), collagen-induced platelet aggregation, and increased spontaneous platelet aggregation were observed. Platelet dysfunction correlated significantly with the degree of proteinuria and hypercholesterolemia (5).
Besides the above described reasons in favor of a hypercoagulable state in nephrotic syndrome, Ozsolylu et al. found that steroids raise the concentration of several clotting factors, principally factor VIII (10). Also Lieberman et al. described that steroids decreased the fibrinolytic activity by incomplete breakdown of the thrombus (11). And diuretics would favor the development of thrombotic complications with their volume depleting effects leading to hemoconcentration.
Other conditions are considered to be contributing factors to hypercoagulability in nephrotic syndrome. These include plasma lipid abnormalities, hypovolemia, hypertension, circulating immune complexes, and susceptibility to infections (12, 13).
Though adult arterial thrombosis is much less common than venous thrombosis in patients with nephrotic syndrome, it is a significant and potentially serious problem due to its high morbidity and mortality. Despite the seriousness, there are few reliable predictors of the individual risk. In addition a propensity for arterial thrombosis may be difficult to demonstrate in adults because of a high baseline incidence and the presence of complicating factors (hypertension, smoking, etc).
In any case, up to now, prophylactic anticoagulation is a difficult problem and remains controversial. Few clinicians would advocate routine anticoagulation in all nephrotic patients. Robert et al. reported that the most important predictive factor for the thrombogenic risk in nephrotic syndrome was the histologic evidence of membranous nephropathy (14). Andrassy et al. suggested that anticoagulation therapy should be administered as long as the patient has a serum albumin level below 2.0 g/dL (1). Bellomo et al. concluded that nephrotic patients with membranous nephropathy should receive prophylactic anticoagulation (15). In 1994 Sarasin and Schifferli suggested that the benefits of prophylactic administration of oral anticoagulants outweigh the risks in nephrotic patients with membranous nephropathy by the Markov-based decision analysis model (16).
Our experience with a case of cerebral infarction in a 53-yr old man with nephrotic syndrome reminded us of prophylactic anticoagulation to reduce the risk of serious arterial thrombosis and suggested that reliable risk factors should be identified.
We reviewed cerebral infarction in adults associated with nephrotic syndrome published by English literature (Table 1). Twelve cases of cerebral infarction in adult nephrotic patients, including this case, have been reported. Male patients were predominant (9 men and 3 women). The mean age was 38.1 yr (range: 21 to 59 yr). The underlying renal disease was membranous nephropathy in three patients, membranoproliferative glomerulonephropathy in three, minimal change disease in three, focal segmental glomerulosclerosis in one and IgA in one, and unknown in one. Steroids and/or diuretics were used in 8 of 11 patients. The mean serum albumin level was 1.45 g/dL (0.7 to 2.7 g/dL). Nine out of twelve patients had severe hypoalbuminemia less than 2.1 g/dL. The serum fibrinogen, antithrombin III, free protein S level in sequence available in 9 of 12, in 11 of 12, and in 7 of 12 of the adult nephrotic patients with cerebral infarction, respectively. Increased fibrinogen levels appeared in six patients and a normal level in three. Six patients showed decreased antithrombin III and serum free protein S was low in four.
Importantly a review of the literature revealed that serious complications such as death occurred in 4 of 12 relatively young patients. Also membranous nephropathy was not predominant; almost all of the cases showed a serum albumin below 2.1 g/dL and arterial thrombosis was more common on steroid and/or diuretics therapy.
In Korean literature, 3 cases of cerebral infarction associated with nephrotic syndrome in adults have been published. The mean age was 24.7 yr (range 23 to 27 yr). There were two patients with minimal change disease, and one with focal segmental glomerulosclerosis. In all cases, steroids and/or diuretics were used and serum albumin levels were below 2.0 g/dL (26-28).
In conclusion, if nephrotic patients have risk factors such as severe hypoalbuminemia and are on diuretics or steroid treatment, even they are young patients with other types of nephrotic syndrome besides membranous nephropathy, prophylactic anticoagulants can be considered to reduce the risk of the serious cerebral infarction. Also, we suggest that reliable risk factors should be further assessed and the potential benefit of prophylactic anticoagulation therapy be examined by decision analysis in nephrotic patients.
Figures and Tables
Fig. 1
MR diffusion image (A) shows a hemorrhagic infarction of the right parieto-occipital area, T2 weighted image (B) shows recent infarction involving the posterior 2/3 portion of the right middle cerebral artery territory and the right head of caudate nucleus.
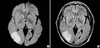
Fig. 2
MR angiography showing a focal luminal narrowing of the bifurcation site of the right middle cerebral artery and right anterior communicating artery and no flow signal intensity in the right internal carotid artery.

Table 1
Summary of adult patients with a cerebral infarction in association with nephrotic syndrome in English literature

*Determined by brain computerized tomographic or magnetic resonance images. †Concurrent with femoral arteries. MCD, minimal change disease; MPGN, membranous proliferative glomerulonephropathy; MGN, membranous glomerulonephropathy; FSGS, focal segmental glomerulosclerosis; MCA, middle cerebral artery; S/D, steroid/diuretics; M, male; F, female.
References
1. Andrassy K, Ritz E, Bommer J. Hypercoagulability in the nephrotic syndrome. Klin Wochenschr. 1980. 58:1029–1036.

2. Kendall AG, Lohmann RC, Dossetor JB. Nephrotic syndrome. A hypercoagulable state. Arch Intern Med. 1971. 127:1021–1027.

3. Kauffmann RH, Veltkamp JJ, Van Tilburg NH, Van Es LA. Acquired antithrombin III deficiency and thrombosis in the nephrotic syndrome. Am J Med. 1978. 65:607–613.

4. Llach F, Koffler A, Finck E, Massry SG. On the incidence of renal vein thrombosis in the nephrotic syndrome. Arch Intern Med. 1977. 137:333–336.

5. Kanfer A, Kleinknecht D, Broyer M, Josso F. Coagulation studies in 45 cases of nephrotic syndrome without uremia. Thromb Diath Haemorrh. 1970. 24:562–571.

6. Kim HJ, Park CH, Kang CM, Park HC, Kim CY, Cho YS. Arterial thrombosis associated with nephrotic syndrome: a case report and review. J Korean Med Sci. 1993. 8:230–234.
7. Vaziri ND, Branson HE, Ness R. Changes of coagulation factors IX, VIII, VII, X, and V in nephrotic syndrome. Am J Med Sci. 1980. 280:167–171.

8. Andrassy K, Oertel PJ, Mehls O, Kreusser W, Koderisch J, Ritz E. Thromboembolic complications and haemostasis in the nephrotic syndrome-- is there a difference between children and adults? Proc Eur Dial Transplant Assoc. 1983. 19:597–601.
9. Vigano-D'Angelo S, D'Angelo A, Kauffman CE Jr, Sholer C, Esmon CT, Comp PC. Protein S deficiency occurs in nephrotic syndrome. Ann Intern Med. 1987. 107:42–47.
10. Ozsoylus S, Strauss HS, Diamone LK. Effects of corticosteroids on coagulation of the blood. Nature (London). 1962. 195:1214–1215.
11. Lieberman E, Heuser E, Gilchrist GS, Donnel GN, Landing BH. Thrombosis, nephrosis and corticosteroid therapy. J Pediatr. 1968. 73:320–328.

13. Cameron JS. Coagulation and thromboembolic complications in the nephrotic syndrome. Adv Nephrol Necker Hosp. 1984. 13:75–114.
14. Robert A, Olmer M, Sampol J, Gugliotta JE, Casanova P. Clinical correlation between hypercoagulability and thrombo-embolic phenomena. Kidney Int. 1987. 31:830–835.

15. Bellomo R, Atkins RC. Membranous nephropathy and thromboembolism: is prophylactic anticoagulation warranted? Nephron. 1993. 63:249–254.

16. Sarasin FP, Schifferli JA. Prophylactic oral anticoagulation in nephrotic patients with idiopathic membranous nephropathy. Kidney Int. 1994. 45:578–585.

17. Parag KB, Somers SR, Seedat YK, Byrne S, Da Cruz CM, Kenoyer G. Arterial thrombosis in nephrotic syndrome. Am J Kidney Dis. 1990. 15:176–177.

18. Marsh EE 3rd, Biller J, Adams HP Jr, Kaplan JM. Cerebral infarction in patients with nephrotic syndrome. Stroke. 1991. 22:90–93.

19. Fuh JL, Teng MM, Yang WC, Liu HC. Cerebral infarction in young men with nephrotic syndrome. Stroke. 1992. 23:295–297.

20. Chaturvedi S. Fulminant cerebral infarctions with membranous nephropathy. Stroke. 1993. 24:473–475.

21. Song KS, Won DI, Lee AN, Kim CH, Kim JS. A case of nephrotic syndrome associated with protein S deficiency and cerebral thrombosis. J Korean Med Sci. 1994. 9:347–350.

23. Ogawa M, Tsukahara T, Saisho H. Nephrotic syndrome with acute renal failure and cerebral infarction in a patient with myasthenia gravis. Am J Nephrol. 1999. 19:622–623.

24. Lee CH, Chen KS, Tsai FC, Chien YY, Lee N. Concurrent thrombosis of cerebral and femoral arteries in a patient with nephrotic syndrome. Am J Nephrol. 2000. 20:483–486.

25. Pandian JD, Sarada C, Elizabeth J, Visweswaran RK. Fulminant cerebral infarction in a patient with nephrotic syndrome. Neurol India. 2000. 48:179–181.
26. Yoon J, Kim CY, Choi MJ, Lim HE, Kim MJ. A case of relapsed minimal-change nephrotic syndrome with multiple brain infarction. Korean J Nephrol. 1991. 10:228–232.
27. Kim YK, Chae DW, Han JS, Kim SK, Lee JS, Han MC, Park JH, Roh JR. Arterial thromboses in nephrotic syndrome. Korean J Nephrol. 1987. 6:153–159.
28. Kang MG, Cho HY, Ryu HK, Lee KM, Bae HK. A case of intracranial arterial thrombosis in nephrotic syndrome. Korean J Nephrol. 1991. 10:224–227.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download