Abstract
Selective introduction of genes conferring chemosensitivity into proliferating tumor cells may be used to treat cancer. We investigated the bystander effect of retrovirusmediated gene transfer of herpes simplex virus thymidine kinase (HSV-TK) gene to murine neuroblastoma cell line (neuro-2a) in vitro and in vivo, and we examined whether the mechanism of bystander effect in neuroblastoma would also depend on connexin-dependent gap junction and/or immune response. A strong bystander effect was observed in vitro, whereby nontransduced tumor cells in proximity to transduced cells acquired susceptibility to ganciclovir (GCV) killing. Implanted mixtures of wildtype cells and HSV-TK transduced cells showed a potent bystander effect upon administration of GCV in A/J mice. HSV-TK/GCV system in murine neuroblastoma induced systemic immunity. Immunohistochemical staining showed many CD4+ and CD8+ cell infiltration but did not show anti-connexin 43+ cells. In conclusion, a strong bystander effect was observed in vitro and in vivo. The bystander effect in murine neuroblastoma might be dependent on immune response and/or on other mechanism such as protein phosphorylation or transfer of apoptotic vesicle, rather than connexin-dependent gap junction.
The herpes simplex virus (HSV) thymidine kinase (TK) gene is a suicide gene under study in both experimental and clinical protocols as a treatment for human cancer. Transfer of the TK gene into tumor cells results in tumor cell death upon exposure to the antiviral prodrug ganciclovir (GCV), as the TK protein phosphorylates the prodrug into a toxic nucleotide analog whose incorporation into nascent DNA results in termination of DNA replication. Despite the in vivo HSV-TK gene transduction of approximately 10-20% of tumor cells, complete tumor eradication occurs in tumors (1). This phenomenon is probably due to a 'bystander effect' associated uniquely with the HSV-TK/GCV system. It includes the transfer of the toxic metabolic products of GCV through gap junction (2), which have recently been shown to be dependent on connexin-mediated intercellular communication (3-5), the phagocytosis of the apoptotic vesicles of dead tumor cells by live tumor cells that mediate apoptosis (6) and the induction of an immune response against the tumor (7-10).
In the present study, we first evaluated the bystander effect in treating neuroblastoma, using HSV-TK transduced neuro-2a cells as a model of neuroblastoma. Second, we examined whether the mechanism of bystander effect in neuro-2a would also depend on connexin-dependent gap junction and/or immune response.
Neuro-2a, a subclone of C1300 murine neuroblastoma A/J mice (American Type Culture Collection, Rockville, MD, U.S.A.), was cultured in modified Eagle medium (GIBCO, Grand Island, NY, U.S.A.) supplemented with 100 µg/mL streptomycin (GIBCO), 100 U/mL penicillin (GIBCO), and with 10% heat-inactivated fetal bovine serum (GIBCO). PA317 is an amphotropic packaging line derived from NIH 3T3 TK- cells transfected with helper plasmid pPAM3. The ecotropic retrovirus packaging cell line PSI-CRE used here contains split helper virus genomes that reduce potential for helper virus generation. Cells were grown in Dulbecco's modified Eagle medium (GIBCO), supplemented with 4.5 g/L glucose and 10% fetal bovine serum.
The HSV-TK gene containing an internal ribosome entry site (IRES) fragment of encephalomyocarditis virus was subcloned in the HpaI site of LNCX retroviral vector as a 1.7-kb BamHI-XhoI insert isolated from the pSXLC-TK after filling-in with Klenow enzyme, resulting in a HSV-TK expressing retroviral vector LNC/IRES/TK. PA317 cells were plated at 1×105 cells per 60-mm dish one day prior to virus exposure. On the day of infection, 1 mL of serially diluted culture medium harvested from the PSI-CRE clonal cell line producing ecotropic virus vector (LNC/IRES/TK) was plated in the presence of 8 µg/mL polybrene for 4 hr. Culture medium was changed and G418-resistant colonies were selected. Virus producing cell lines producing high-titer (>1×107 cfu/mL) retroviral vectors were designated PA317/LNC/IRES/TK and were used as sources of recombinant retroviral vectors for transduction of neuro-2a cells.
For in vitro infection, neuro-2a cells were incubated for 4 hr at 37℃ in the presence of 8 µg/mL polybrene with filtered supernatant from retroviral producer cells (PA317/LNC/IRES/TK). After the supernatant was changed with fresh medium, the cells were incubated for another 48 hr before changing into selection media that contained 500 µg/mL G418 (Geneticin, GIBCO). Isolated clones were picked 14 days later, analyzed for sensitivity to GCV (Cymevene, Syntex Laboratories, Inc., Palo Alto, CA, U.S.A.) and were designated neuro-2a/TK. For the in vitro HSV-TK bystander assay, 1×106 cells were plated in triplicate into 6-well plates as follows: 100% transduced and 100% untransduced cells and mixtures of transduced and untransduced cells at ratios of 1:1 and 1:10, and 1:20. After 24 hr of incubation, cells were exposed to 10 µg/mL GCV in medium. Medium was exchanged every other day. On day 1, day 2, day 3 and day 4, surviving cells were counted by trypan blue exclusion method.
A/J mice, 6-10 weeks of age, were obtained from Yonsei Clinical Research Center (Seoul, Korea). For tumor cell implantation, cells were enzymatically detached from culture flasks and counted. 2×106 cells were resuspended in 100 µL of serum-free medium and were injected subcntaneously (s.c.) into the flank as follows: 100% HSV-TK-transduced (Group 1), 100% untransduced cells (Group 2) and mixtures of transduced and untransduced cells at ratios of 1:1 (Group 3), 1:10 (Group 4) and 1: 20 (Group 5). Tumor measurements were performed at least once a week with calipers. When the tumors reached the diameter of 5-8 mm, the animals were randomly assigned to treatment groups (N=8/group), and the mice received 50 mg/kg GCV intraperitoneally twice a day for 7 days. Animals were monitored for 50 days.
To examine the induction of an immune response against the tumor, 2×106 HSV-TK cells and 2×106 neuro-2a cells injected s.c. into the flank of the mice, respectively (N=6/group). Seven days after tumor cells challenge, the mice received 50 mg/kg GCV intraperitoneally twice a day for 7 days, and then the animals were challenged by s.c. implantation of approximately 2×106 unmodified neuro-2a cells contralaterally to the original injection sites 14 days after the last GCV treatment. The survival rate was monitored weekly for 50 days. Statistical analysis was done by log rank test.
In Group 5, the tumor which was established with 25% HSV-TK-positive cells were dissected, embedded in paraffin, and were cut into 4 µm sections. We stained the tumor tissue with mouse anti-connexin 43 monoclonal antibody (Chemicon International, Inc., Temecula, CA, U.S.A.). And the subcutaneous tissue of the tumor-regressed site which was injected with 25% HSV-TK-positive cells and followed by GCV treatment were dissected after the tumor reduced completely and the sample were embedded in paraffin, cut into 4 µm sections. The primary antibodies used in the immunohistochemistry were goat polyclonal antibodies to cell membrane of CD4, rabbit polyclonal antibodies to cell membrane of CD8 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, U.S.A.). The slide were stained with horseradish-peroxidase-linked streptavidin (DAKO, Carpinteria, CA, U.S.A.), and then counterstained by Mayer hematoxylin.
The survival of the mixtures containing 0, 5, 10, 50, 100% HSV-TK-positive cells which were incubated with 10 µg/mL GCV for 4 days was 142%, 53%, 0%, 0%, respectively. These findings suggest that the bystander effect is significant in HSV-TK-expressing cells (Fig. 1).
To assess the bystander effect, when the tumors reached the diameter of 5-8 mm, the animals were randomly assigned to treatment groups, and the mice received 50 mg/kg GCV intraperitoneally twice a day for 7 days. In Groups 1 and 3, the sizes of the tumors decreased when followed by a course of GCV, and all tumors regressed completely at 5 days and at 7 days after GCV treatment, respectively. The survival rate at 50 days was 100% in both groups. In Group 4, the growth of the tumor delayed during the GCV treatment and the survival rate at 50 days was 25% (Fig. 2).
None of the mice, which treated by GCV after 100% HSV-TK cell injection and received the second tumor cell challenges, developed the tumor at the site of challenge of unmodified neuro-2a cells during the observation period. In contrast, all of the mice of the control group developed tumors. These results support that the death of HSV-TK positive cells by GCV treatment elicit an anti-tumor immune response (Fig. 3).
With current gene transfer techniques, more than 10% transfection efficiency can be obtained in the vicinity of the gene transfer site in vivo (11). However, the overall transfection efficiency in human tumor models is still very low because the number of cells in the vicinity of the transfection site is low compared with the total tumor mass (11). Accordingly bystander effect in HSV-TK gene therapy gives great advantages in tumor treatment.
In this study we used the syngenic neuroblastoma model in A/J mice. Mice in Groups 1 and 3 were followed for up to 100 days without tumor recurrence. Significantly prolonged survival in animals which were injected with more than 50% HSV-TK-positive cells and followed by GCV treatment was observed. Our results show that, both in vitro and in vivo, more than 10% HSV-TK-transduced cells among wild type neuro-2a cells are required for a beneficial GCV treatment effect. The results are in line with other tumor models previously published (3, 12, 13) and indicate that the bystander effect also occurs in the murine neuroblastoma cells. Our results confirm the important role of the bystander effect in the treatment of experimental neuroblastoma. This bystander effect has important clinical implications, in that the HSV-TK gene need not be delivered to all malignant cells and may operate over a distance. However, there are also reports indicating that HSV-TK gene transfer followed by GCV treatment does not lead to a complete eradication of other experimental tumor (14). The reason for the discrepancy in HSV-TK/GCV results is currently unclear, but may involve different cell lines or conditions used for the gene transfer and GCV treatment. Also, the differences in tissue reactions in various experimental tumor models probably affect the final outcome (15-17).
The mechanism of the bystander effect is still under investigation. It appears to be enhanced by cell-to-cell contact and by the presence of gap junctions, which are composed of connexin, a family of homologous proteins expressed in cell- and tissue-specific patterns. There are close correlations between tumor cell gap junctional intercellular communication and the magnitude of the HSV-TK bystander effect. Tumors not expressing connexin at all will be ineffective in bystander effect (18, 19). Indeed, the bystander effect is likely to be phosphorylated GCV that enters TK-cells through cellular junction. The uptake of phosphorylated GCV released from apoptotic cells may also contribute to the bystander effect. Apoptosis seems to be an important mechanism for cell death, as also demonstrated by others (20, 21). However, Dahle et al. (22) reported that bystander effect was not mediated by gap junctional intercellular communication but protein phosphorylation is important in cellular signaling pathways and may be involved in the bystander effect, for example by influencing the mode of cell death. In our experiment we did not observed the connexin 43 expression on murine neuroblastoma model. Andrade-Rozental et al. (23) also demonstrated that neuro-2a cells are expressed very low endogenous level of gap junctional communication. This result indicates that the bystander effect in murine neuroblastoma depends on other mechanism such as the phagocytosis of the apoptotic vesicles of dead tumor cells by live tumor cells or the induction of an immune response against the tumor rather than connexin-dependent gap junction.
We have shown that significant anti-tumor immunity does develop following HSV-TK and GCV treatments of murine neuroblastoma. Barba et al. (9) suggested that HSV-TK and GCV treatment of brain tumor induced the significant antitumor immune response. We suggest that, in immunogenic tumor, the immune system participates in maintaining longterm tumor regression and induces the development of bystander effect (24-26). The development of anti-tumor immunity following HSV-TK and GCV treatment could be used in tumor vaccination strategies. The emergence of a protective immunity against the parental tumor cells might be with the appearance of tumor-specific cytotoxic T cells or APCs derived from mice following HSV-TK and GCV treatment and continue for a long time.
These observations suggest the use of cytokines to the natural immune response as a potential cancer therapy, and the long-term survival could be improved by stimulation of an immune response toward inoculated tumor cells (12). In the future the stimulation of inflammatory and immunological reactions by combined gene transfer of HSV-TK and cytokines such as interleukin-2 (IL-2), IL-3, IL-12, or granulocyte macrophage colony stimulating factor might improve the effectiveness of the HSV-TK/GCV treatment in neuroblastoma model, especially when only low transfection efficiencies can be obtained.
Figures and Tables
Fig. 1
In vitro bystander assay. Wild type neuro-2a cells and neuro-2a/TK cells were mixed at varying ratios, plated in 6 well culture plates (106/cell), and incubated at 37℃ overnight. Cells were exposed to GCV (10 µg/mL). On day 1, day 2, day 3 and day 4, cells were stained with trypan blue and surviving cells were enumerated.

Fig. 2
In vivo bystander effect. 2×106 cells were injected s.c. into the flank as follows: 100% HSV-TK-transduced (Group 1) and 100% untransduced cells (Group 2) and mixtures of transduced and untransduced cells at ratios of 1:1 (Group 3), 1:10 (Group 4) and 1:20 (Group 5). When the tumors reached the diameter of 5-8 mm, the mice received intraperitoneally 50 mg/kg GCV twice a day for 7 days. Group 2 versus Group 1: significant, p<0.01; Group 2 versus group 3: significant, p<0.01; Group 2 versus Group 4: significant, p<0.01; Group 2 versus Group 5: significant, p<0.05 (log rank test).

Fig. 3
Induction of immune response in HSV-TK gene-transduced neuro-2a cells. 2×106 HSV-TK cells (Group 1) and 2×106 neuro-2a cells (Group 2) injected s.c. into the flank of the mice, respectively (N=6/group). One week after tumor cell challenge, the mice received intraperitoneally 50 mg/kg GCV twice a day for 7 days and then the animals were challenged by s.c. implantation of approximately 2×106 unmodified neuro-2a cells contralaterally to the original injection sites 14 days after the last GCV treatment. Log rank test showed group survived significantly compared with Group 2 (p<0.01).
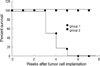
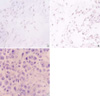
Fig. 4
Expression of CD 4, CD 8 and connexin-43 antigen. Paraffin-embedded sections were reacted with goat polyclonal antibodies to CD4 (A), rabbit polyclonal antibodies to CD8 (B) and not reacted with mouse anti-connexin 43 antibody (C). (A, B) Cell membrane staining of subcutaneous tissue of the tumor-regressed site. Magnification was at ×1,000. (C) Solid area of neuroblastoma before GCV. Magnification was at ×400.
References
1. Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL, Abraham GN. The bystander effect: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993. 53:5274–5283.
2. Touraine RL, Ishii-Morita H, Ramsey WJ, Blaese RM. The bystander effect in the HSVtk/ganciclovir system and its relationship to gap junctional communication. Gene Ther. 1998. 5:1705–1711.

3. Estin D, Li M, Spray D, Wu JK. Connexins are expressed in primary brain tumors and enhance the bystander effect in gene therapy. Neurosurgery. 1999. 44:361–368.

4. Duflot-Dancer A, Piccoli C, Rolland A, Yamasaki H, Mesnil M. Longterm connexin-mediated bystander effect in highly tumorigenic human cells in vivo in herpes simplex virus thymidine kinase/ganciclovir gene therapy. Gene Ther. 1998. 5:1372–1378.

5. McMasters RA, Saylors RL, Jones KE, Hendrix ME, Moyer MP, Drake RR. Lack of bystansder killing in herpes simplex virus thymidine kinase-transduced colon cell lines due to deficient connexin 43 gap junction formation. Hum Gene Ther. 1998. 9:2253–2261.
6. Colombo BM, Benedetti S, Ottolenghi S, Mora M, Pollo B, Poli G, Finocchiaro G. The bystander effect: Association of U-87 cell death with Ganciclovir-mediated apoptosis of nearby cells and lack of effect in athymic mice. Hum Gene Ther. 1995. 6:763–772.

7. Vile RG, Nelson JA, Castleden S, Chong H, Hart IR. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994. 54:6228–6234.
8. Caruso M, Panis Y, Gagandeep S, Houssin D, Salzmann JL, Klatzmann D. Regression of established macroscopic liver metastases after in situ transduction of a suicide gene. Proc Natl Acad Sci USA. 1993. 90:7024–7028.

9. Barba D, Hardin J, Sadelain M, Gage FH. Development of anti-tumor immunity following thymidine kinase-mediated killing of experimental brain tumors. Proc Natl Acad Sci USA. 1994. 91:4348–4352.

10. Ramesh R, Munshi A, Abboud CN, Marrogi AJ, Freeman SM. Expression of costimulatory molecules: B7 and ICAM up-regulation after treatment with a suicide gene. Cancer Gene Ther. 1996. 3:373–384.
11. Puumalainen AM, Vapalahti M, Agrawal RS, Kossila M, Laukkanen J, Lehtolainen P, Viita H, Paljarvi L, Vanninen R, Yla-Herttuala S. Beta- galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther. 1998. 9:1769–1774.
12. Sandmair AM, Turunen M, Tyynela K, Loimas S, Vainio P, Vanninen R, Vapalahti M, Bjerkvig R, Janne J, Yla-Herttuala S. Herpes simplex virus thymidine kinase gene therapy in experimental rat BT4C glioma model: effect of the percentage of thymidine kinase-positive glioma cells on treatment effect, survival time, and tissue reactions. Cancer Gene Ther. 2000. 7:413–421.

13. Walling HW, Swarthout JT, Culver KW. Bystander-mediated regression of osteosarcoma via retroviral transfer of the herpes simplex virus thymidine kinase and human interleukin-2 genes. Cancer Gene Ther. 2000. 7:187–196.

14. Tapscott SJ, Miller AD, Olson JM, Berger MS, Groudine M, Spence AM. Gene therapy of rat 9L gliosarcoma tumors by transduction with selectable genes does not require drug selection. Proc Natl Acad Sci USA. 1994. 91:8185–8189.

15. Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998. 36:91–102.
16. Ram Z, Walbridge S, Shawker T, Culver KW, Blaese RM, Oldfield EH. The effect of thymidine kinase transduction and ganciclovir therapy on tumor vasculature and growth of 9L gliomas in rats. J Neurosurg. 1994. 81:256–260.

17. Ramesh R, Marrogi AJ, Munshi A, Abboud CN, Freeman SM. In vivo analysis of the 'bystander effect': a cytokine cascade. Exp Hematol. 1996. 24:829–838.
18. Naus CC, Bechberger JF, Zhang Y, Venance L, Yamasaki H, Juneja SC, Kidder GM, Giaume C. Altered gap junctional communication, intercellular signaling, and growth in cultured astrocytes deficient in connexin 43. J Neurosci Res. 1997. 49:528–540.
19. Marconi P, Tamura M, Moriuchi S, Krisky DM, Niranjan A, Goins WF, Cohen JB, Glorioso JC. Connexin 43-enhanced suicide gene therapy using herpesviral vectors. Mol Ther. 2000. 1:71–81.

20. Samejima Y, Meruelo D. 'Bystander killing' induces apoptosis and is inhibited by forskolin. Gene Ther. 1995. 2:50–58.
21. Hamel W, Magnelli L, Chiarugi VP, Israel MA. Herpes simplex virus thymidine kinase/ganciclovir-mediated apoptotic death of bystander cells. Cancer Res. 1996. 56:2697–2702.
22. Dahle J, Mikalsen SO, Rivedal E, Steen HB. Gap junctional intercellular communication is not a major mediator in the bystander effect in photodynamic treatment of MDCK II cells. Radiat Res. 2000. 154:331–341.

23. Andrade-Rozental AF, Rozental R, Hopperstad MG, Wu JK, Vrionis FD, Spray DC. Gap junctions: the "kiss of death" and the "kiss of life". Brain Res Rev. 2000. 308–315.

24. Agard C, Ligeza C, Dupas B, Izembart A, El Kouri C, Moullier P, Ferry N. Immune-dependent distant bystander effect after adenovirus-mediated suicide gene transfer in a rat model of liver colorectal metastasis. Cancer Gene Ther. 2001. 8:128–136.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download