Abstract
Background
This study aimed to investigate the effect of strenuous exercise on β-endorphine (β-END) level in fibromyalgia (FM) patients compared to healthy subjects.
Methods
We enrolled 30 FM patients and 15 healthy individuals. All study participants underwent a treadmill exercise test using modified Bruce protocol (M.Bruce). The goal of the test was achieving at least 70% of the predicted maximal heart rate (HRMax). The serum levels of β-END were measured before and after the exercise program. Measurements were done while heart rate was at least 70% of its predicted maximum.
Results
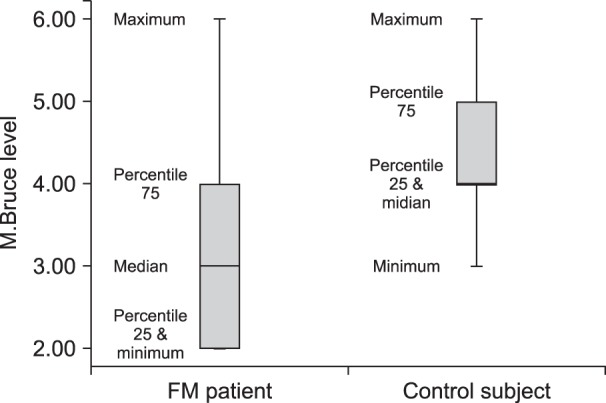
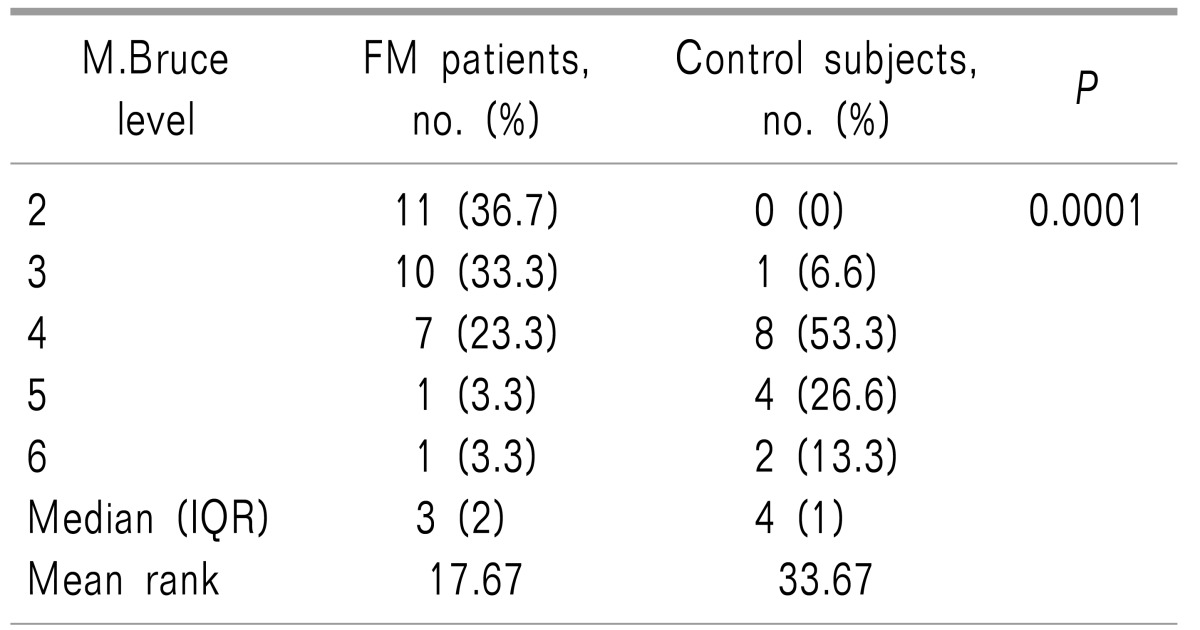
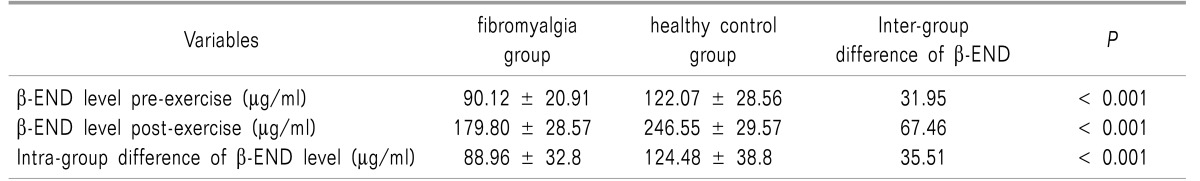
The mean ± the standard deviation (SD) of exercise duration in the FM and control groups were 24.26 ± 5.29 and 29.06 ± 3.26 minutes, respectively, indicating a shorter time to achieve the goal heart rate in FM patients (P < 0.003). Most FM patients attained 70% HRMax at lower stages (stage 2 and 3) of M.Bruce compared to the control group (70% versus 6.6%, respectively; P < 0.0001). Compared to healthy subjects, FM patients had lower serum β-END levels both in baseline and post-exercise status (Mean ± SD: 122.07 ± 28.56 µg/ml and 246.55 ± 29.57 µg/ml in the control group versus 90.12 ± 20.91 µg/ml and 179.80 ± 28.57 µg/ml in FM patients, respectively; P < 0.001).
Aerobic exercise has been increasingly known as the most effective intervention in FM treatment [12]. It stimulates the secretion of endorphins/enkephalins and noradrenergic–serotonergic neurotransmitters [345] and is associated with reduced pain perception and a higher pain threshold [6].
β-END has a putative role in the attenuation of nocioception signaling in ascending pain pathways [78]. This analgesic effect, however, has been observed to be decreased in FM [9]. According to a plausible hypothesis, nociceptive signaling is more likely to gain access to the brain in patients with FM owing to impaired inhibitory control at the level of the spinal cord [910]. While an interest in the role of β-END in FM surged in the mid-1980s, it soon waned as initial studies were unable to show any significant difference in serum or cerebrospinal fluid (CSF) β-END levels between FM patients and healthy subjects [111213]. However, subsequent data demonstrated high levels of enkephalins, another main endogenous peptide, in the CSF of FM patients [14]. This paradoxical observation may indicate a failed attempt by central pathways to control nociception in fibromyalgia [914].
The existing data on the endogenous opioid pain mediation in FM and other chronic pain disorders has been mainly derived from settings where basal (resting) levels of endogenic opioid neuropeptides were investigated. As FM represents a condition of continuous distress, imposing a challenge to inhibitory opiate neural pathways, research on stress models may be more sensitive in disclosing the endorphin derangement in FM.
In this study, we considered strenuous physical exercise to be analogous to a stressful condition. Since hypothetically a difference between FM and healthy subjects' β-END levels is more probable after exercise, we raised this question, "How would endorphin levels change after exercise in FM patients and healthy subjects?"
This study was done at the Iranian Academic Center for Education, Culture and Research (ACECER). Enrolled FM patients aged between 18 and 60 years, and met the 1990 American College of Rheumatology Criteria (ACR). Healthy participants, however, were selected from volunteer healthy members of the FM patients' family. Of note, these healthy participants were all gender-matched and met age inclusion criteria.
On the other hand, we excluded patients who had used psychotropic drugs, opiates or hormonal replacement therapy within the past 8 weeks. All subjects underwent a clinical examination by one rheumatologist, and if they had abnormal clinical findings, cardiopulmonary disorders, physical impairment, or inability to exercise, they were excluded from the study as well.
All participants were asked to fill out the demographic characteristics form. Then, a 15-20 minute physical rest in supine position was offered to each person for achieving their basal heart rate (60 to 100 per minute). Then, a baseline blood sample was drawn from an anti-cubital vein. Another blood sample was taken at the end of the exercise schedule that had been arranged according to the modified protocol of Bruce et al. [15]. The level of exercise intensity was recorded in each subject when the heart rate reached 70% of the predicted maximal value (HRMax).
Written informed consent was obtained from all the participants, and the study was approved by the Ethics Committee of ACECER.
All study participants underwent a treadmill exercise test using modified Bruce protocol [16]. Briefly, the protocol consists of 2 warm-up stages each lasting 3 minutes. The first is at 1.7 mph and a 0% grade, and the second is at 1.7 mph and a 5% grade. At three minute intervals the incline of the treadmill increases by 2%, and the speed increases in accordance to the 10-stage protocol. The test is stopped when the subject cannot continue running due to fatigue, pain, or physical exhaustion. All subjects were continuously monitored for heart rate, blood pressure and cardiac electrical activity. When the goal of the heart rate, 70% of the predicted heart rate maximum (HRMax), was achieved at any level of exercise testing, the subject was asked to continue the exercise for an additional 15 minutes. Then, after stopping exercise on the treadmill, the second blood sample was drawn when the heart rate was still ≥ 70% of the HRMax.
The sera were stored at -20℃ until analyzed. The tubes containing the serum samples were number-coded in order to blind the laboratory personnel and the sequence of sample collection. Analytical measurements of serum β-END were accomplished by high performance liquid chromatography (HPLC) with colorimetric detection. Briefly, the sample was redissolved in mobile phase and injected into a high performance liquid chromatography column for analysis. The mobile phase contained trifluoroacetic acid and acetonitrile. The flow rate was 1 ml per minute through Vydac RPC-18 250 × 4.6 µm column (Discovery Bio wide Pore C18 5 µm [supelco]). Colorimetric detection was accomplished with electrochemical cells in series (schimadzu LC-9AUV) with a pressure of 160 psi. β-END concentration was then determined by comparison of the samples' peak areas with the extracted standard's peak area (β-END humor with a molecular weight of 3462.63-3468.63 from Sigma-Aldrich Company, USA). Final concentrations were eventually calculated relative to identically extracted internal standard of known concentration.
An independent Student t-test was used to compare the mean of β-END concentration in FM patients and in the control individuals. Similarly, a paired t-test was used to compare intra-group β-END concentration means before and after the exercise. Finally, Mann-Whitney analysis was performed to compare the M.Bruce stage in the two groups. Mean differences were considered to be statistically significant when P < 0.05. All statistical analyses were carried out using SPSS for Windows version 17.0 (IBM, Armonk, NY, USA).
All study participants were female including the 30 FM patients and the 15 healthy subjects. Two patients from FM group failed to run for the extra 15 minutes after achieving 70% of their HRMax and left the M.Bruce protocol because of exhaustion.
The mean ± SD age of the FM patients was significantly greater than that of the control group (41.7 ± 10.26 versus 30.67 ± 4.13 years, respectively; P < 0.001).
Mean ± SD exercise duration in the FM and in the control groups (including the additional 15 minutes after attaining 70% of HRMax) were 24.26 ± 5.29 and 29.06 ± 3.26 minutes, respectively, indicating a shorter time to achieve to goal heart rate in the FM patients (P < 0.003). As illustrated in Table 1, most FM patients attained 70% of their HRMax at the stage 2 or 3 of the M.Bruce protocol (36.7% and 33.3% of total FM patients, respectively). Only two FM patients (6.6%) achieved 70% of their HRMax at higher stages (level of 5 and 6) of the M.Bruce protocol. Conversely, most healthy subjects reached HRMax in higher stages of exercise. In fact, 93% of control subjects reached stage 4 (versus 30% of FM patients) and 40% (6 persons) achieved 70% of their HRMax in stages 5 and 6 (Fig. 1).
As shown in Table 2, FM patients had lower serum β-END levels both in baseline and post-exercise status than healthy subjects. Our data also demonstrated that exercise increased serum β-END level in both groups but the average rise in β-END in FM patients was significantly lower than in the control group (Table 2).
This study revealed that FM patients had lower baseline β-END levels than healthy individuals. Moreover, a blunted surge response of β-END to exercise was observed in FM patients. It was also shown that FM patients reached the predicted maximal heart rate in lower stages of exercise (stage 2 and 3 of modified Bruce protocol) compared to healthy subjects.
Initial studies examining basal serum β-END levels in the FM population have been contradictory. For instance, Yunus et al. [11] and Hall et al. [12] reported that serum β-END levels were not different between FM patient and healthy individuals. However, this finding was debated in the following studies. Panerai et al. [16] showed that mononuclear cells' β-END level that probably reflects central β-END concentrations better than plasma concentrations, was lower in FM patients than in healthy subjects. The results of this study are congruent with the previous data showing that concentration of the opioid β-END is lower in FM patients in comparison with normal subjects. This lower opioid tone may be a key factor in the development of chronic allodynia and the increased sensitization to peripheral stimuli seen in FM patients.
Although there were a few reports regarding the CSF resting level or serum level of β-END in FM patients, the impact of stress inducers such as physical exercise on serum β-END had not been previously studied. In healthy subjects, it is known that aerobic physical activity is capable of increasing peripheral levels of β-END, promoting a decrease in sympathetic activity, improving sleep, and facilitating psychological stability [517181920]. It appears that exercise acts upon many pain mechanisms such as the serotonergic system [21], sympathetic activity [18], and β-END [17]. Even so, in fibromyalgia patients, it seems that the positive results of aerobic exercise may be diminished at least partly due to failure of normal elevation of β-END. Our data supported this hypothesis and inter-group comparison of serum β-END levels demonstrated that although exercise significantly increased serum β-END levels in both groups, this effect was less prominent in the FM group. This observation might explain why FM patients have more post-exercise muscle pain and tenderness or fatigue; this fact, if confirmed, may be one step ahead towards understanding the mechanism of pain and diffuse hyperalgesia in fibromyalgia.
It has been previously shown that autonomic dysfunction may be involved in the heart rate's variable response during and after exercise in FM patients [22]. Interestingly, we found that FM patients required lower stages of graded exercise to achieve the age-predicted maximal heart rate. We think that such exercise intolerance in these patients could be related to the inappropriate response of their heart rate to exercise and the less favorable cardiovascular fitness of FM patients that probably occurs in the context of autonomic dysfunction. Decrease in exercise capacity and duration in FM patients can lead to failure of β-END and other endogenous opiates to rise normally after exercise. Consequently, it is conceivable that pain and fatigue after exercise could be impacted by autonomous dysfunction and lower exercise tolerance. This relationship may be further elucidated when our understanding of the changes in endogenous opiates during and after exercise improves.
Although physical exercise is widely acknowledged as a fundamental management for FM [12], there are very few studies exploring the biochemical mechanisms happening during exercise; therefore, the mechanisms responsible for the achieved benefits of exercise remain to be explained.
This is the first study to assess the β-END response to exercise in FM patients. Our results may guide future research in unifying the concepts of post-exercise symptoms and autonomic dysfunction seen in FM patients. Much interest has recently been expressed in the possible role of the autonomic nervous system in the pathogenesis of chronic pain and fatigue syndromes like FM [22]. In this regard, it has been suggested that abnormal sympathetic activation could be involved in the pathogenesis of chronic pain and fatigue syndromes in FM [23].
This study was limited mainly by its small sample size. Furthermore, participants of both groups were not aged-matched. As the previous studies [242526] showed that β-END levels are negatively correlated with age, it means that the basal β-END levels in our study may be influenced by age and the higher mean age of the FM patients might have contributed to the difference of basal β-END levels between the FM group and healthy group. It is an important issue in the interpretation of our data, but it is worthy of special attention that β-END levels rising was blunted after exercise in FM patients rather than control group. There is no study in the literature which showed the effect of age on post exercise β-END levels. So, we postulated that β-END level differences between the FM group and healthy group are more than only "age effects". Moreover, we did not evaluate the possibility that the FM patients had had concurrent comorbid psychiatric syndromes such as depression that could have changed their serum β-END level [24]. Also, we used peripheral values of β-END in our study. It is important to note that the serum level of β-END may not reflect exactly its central activity. In fact, the degree to which peripheral values of β-END reflect its central activity is an ever-present challenge to clinical researchers. However, the data provide a compelling argument for the routine measurement of plasma β-END [242728].
In summary, we found that FM patient had lower levels of β-END in both basal and post-exercise states. It could be related to autonomic dysfunction, lower levels of cardiovascular fitness or to endogenous central dysfunction. It remains to be explained how exercise exerts its beneficial effects on FM patients. In addition, it should further be studied whether autonomic system training by exercise could improve β-END levels or have a positive influence on the other pathologic mechanisms involved in FM. These answers can lead to the improvement in our understanding of neurobiological mechanisms in FM.
References
1. Nüesch E, Häuser W, Bernardy K, Barth J, Jüni P. Comparative efficacy of pharmacological and non-pharmacological interventions in fibromyalgia syndrome: network meta-analysis. Ann Rheum Dis. 2013; 72:955–962. PMID: 22739992.

2. Lauche R, Häuser W, Jung E, Erbslöh-Möller B, Gesmann M, Kühn-Becker H, et al. Patient-related predictors of treatment satisfaction of patients with fibromyalgia syndrome: results of a cross-sectional survey. Clin Exp Rheumatol. 2013; 31:S34–S40. PMID: 23710561.
3. Angelopoulos TJ, Denys BG, Weikart C, Dasilva SG, Michael TJ, Robertson RJ. Endogenous opioids may modulate catecholamine secretion during high intensity exercise. Eur J Appl Physiol Occup Physiol. 1995; 70:195–199. PMID: 7607192.

4. Jonsdottir IH, Hoffmann P, Thorèn P. Physical exercise, endogenous opioids and immune function. Acta Physiol Scand Suppl. 1997; 640:47–50. PMID: 9401605.
5. Schwarz L, Kindermann W. Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med. 1992; 13:25–36. PMID: 1553453.

6. Koltyn KF, Garvin AW, Gardiner RL, Nelson TF. Perception of pain following aerobic exercise. Med Sci Sports Exerc. 1996; 28:1418–1421. PMID: 8933493.

7. Goldfarb AH, Jamurtas AZ. Beta-endorphin response to exercise. An update. Sports Med. 1997; 24:8–16. PMID: 9257407.
8. Paulev PE, Thorbøll JE, Nielsen U, Kruse P, Jordal R, Bach FW, et al. Opioid involvement in the perception of pain due to endurance exercise in trained man. Jpn J Physiol. 1989; 39:67–74. PMID: 2542682.

9. Schmidt-Wilcke T, Clauw DJ. Fibromyalgia: from pathophysiology to therapy. Nat Rev Rheumatol. 2011; 7:518–527. PMID: 21769128.

10. Vierck CJ Jr, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain. 2001; 2:334–344. PMID: 14622813.

11. Yunus MB, Denko CW, Masi AT. Serum beta-endorphin in primary fibromyalgia syndrome: a controlled study. J Rheumatol. 1986; 13:183–186. PMID: 2939238.
12. Hall S, Littlejohn GO, Jetwa J, Copocolor D. Plasma beta-endorphin levels in fibrositis. Arthritis Rheum. 1983; 26:S539.
13. Vaeroy H, Helle R, Førre O, Kåss E, Terenius L. Cerebrospinal fluid levels of beta-endorphin in patients with fibromyalgia (fibrositis syndrome). J Rheumatol. 1988; 15:1804–1806. PMID: 2466120.
14. Baraniuk JN, Whalen G, Cunningham J, Clauw DJ. Cerebrospinal fluid levels of opioid peptides in fibromyalgia and chronic low back pain. BMC Musculoskelet Disord. 2004; 5:48. PMID: 15588296.

15. Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973; 85:546–562. PMID: 4632004.

16. Panerai AE, Vecchiet J, Panzeri P, Meroni P, Scarone S, Pizzigallo E, et al. Peripheral blood mononuclear cell beta-endorphin concentration is decreased in chronic fatigue syndrome and fibromyalgia but not in depression: preliminary report. Clin J Pain. 2002; 18:270–273. PMID: 12131069.

17. Droste C, Greenlee MW, Schreck M, Roskamm H. Experimental pain thresholds and plasma beta-endorphin levels during exercise. Med Sci Sports Exerc. 1991; 23:334–342. PMID: 2020272.

18. Thorén P, Floras JS, Hoffmann P, Seals DR. Endorphins and exercise: physiological mechanisms and clinical implications. Med Sci Sports Exerc. 1990; 22:417–428. PMID: 2205777.
19. Torsvall L, Akerstedt T, Lindbeck G. Effects on sleep stages and EEG power density of different degrees of exercise in fit subjects. Electroencephalogr Clin Neurophysiol. 1984; 57:347–353. PMID: 6200299.

20. Janal MN, Colt EW, Clark WC, Glusman M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain. 1984; 19:13–25. PMID: 6330643.

21. Valim V, Natour J, Xiao Y, Pereira AF, Lopes BB, Pollak DF, et al. Effects of physical exercise on serum levels of serotonin and its metabolite in fibromyalgia: a randomized pilot study. Rev Bras Reumatol. 2013; 53:538–541. PMID: 24477734.

22. Meeus M, Goubert D, De Backer F, Struyf F, Hermans L, Coppieters I, et al. Heart rate variability in patients with fibromyalgia and patients with chronic fatigue syndrome: a systematic review. Semin Arthritis Rheum. 2013; 43:279–287. PMID: 23838093.

23. Martinez-Lavin M. Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia. Arthritis Res Ther. 2007; 9:216. PMID: 17626613.

24. Hegadoren KM, O'Donnell T, Lanius R, Coupland NJ, Lacaze-Masmonteil N. The role of beta-endorphin in the pathophysiology of major depression. Neuropeptides. 2009; 43:341–353. PMID: 19647870.

25. Nagamitsu S, Matsuishi T, Komori H, Yamashita Y, Eguchi H, Ichikawa K, et al. Age-related changes in the cerebrospinal fluid level of beta-endorphin and substance P. Short communication. J Neural Transm (Vienna). 1998; 105:53–58. PMID: 9588760.

26. Akil H, Haskett RF, Young EA, Grunhaus L, Kotun J, Weinberg V, et al. Multiple HPA profiles in endogenous depression: effect of age and sex on cortisol and betaendorphin. Biol Psychiatry. 1993; 33:73–85. PMID: 8382535.

27. King M, Su W, Chang A, Zuckerman A, Pasternak GW. Transport of opioids from the brain to the periphery by P-glycoprotein: peripheral actions of central drugs. Nat Neurosci. 2001; 4:268–274. PMID: 11224543.

28. Ghavidel-Parsa B, Bidari A, Amir Maafi A, Ghalebaghi B. The iceberg nature of fibromyalgia burden: the clinical and economic aspects. Korean J Pain. 2015; 28:169–176. PMID: 26175876.





 Citation
Citation Print
Print


 XML Download
XML Download