This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Adenosine has been shown to have a wide spectrum of unique pain-relieving effects in various clinical situations. The aim of this study was to investigate the effects of intraoperative adenosine infusion on acute opioid tolerance and opioid induced hyperalgesia induced by remifentanil in adult patients undergoing tonsillectomy.
Methods
For this study, ninety patients were randomly allocated into groups that receive either adenosine (adenosine group) or saline (remifentnail group) intravenously under remifentanil based anesthesia and saline (sevoflurane group) under sevoflurane anesthesia. The patients in adenosine group received adenosine at dose of 80 µg/kg/min, and those in remifentnail group and sevoflurane group received an equal volume of saline 10 minutes after the induction of anesthesia until the end of surgery. Intraoperative evaluation included time weighted mean remifentanil dose, and postoperative evaluations included degree of pain severity at 1, 6, 12, and 24 hours, time to first postoperative requirement, and analgesic dose required during 24 hours after operation.
Results
Time weighted mean remifentanil dose during intraoperative period in adenosine group was significantly lower than that of remifentnail group (P = 0.00). The first postoperative analgesic were required earlier in remifentanil group than sevoflurane group or adenosine group (P = 0.00). Pethidine requirement during 24 hours in sevoflurane group and adenosine group was significantly lower than that of remifentnail group (P = 0.00). The visual analog scale scores for pain in sevoflurane group and adenosine group were significantly lower than those of remifentnail group for 12 hours after operation (P = 0.00). Incidence of hypotension (P = 0.024) and number of ephedrine administered (P = 0.011) in adenosine group were significantly higher than those of sevoflurane group.
Conclusions
The above results suggest that intraoperative adenosine infusion prevent acute opioid tolerance and opioid induced hyperalgesia induced by remifentanil.
Keywords: adenosine, analgesic requirement, opioid induced hyperalgesia, remifentanil
INTRODUCTION
Adenosine, one of purine compounds plays as significant roles in central and peripheral nociception/pronociception or antinociception/analgesia have been extensively reviewed [
1-
3]. Intraoperative adenosine infusion reduces inhalation anesthetic requirements and postoperative analgesic requirements in patients undergoing various surgical procedures [
3-
6]. Also adenosine infusion at a non-hypotensive low dose (80 µg/kg/min) during surgery reduced the inhalational anesthetic requirements by 20-50% and kept intraoperative systolic blood pressure levels more stable and less responsive to painful surgical stimuli in the adenosine group than in the placebo group. After breast surgery and hysterectomy, postoperative opioid requirements during the first 24 h were reduced by 27% and 18%, respectively, at a similar degree of pain relief, suggesting an extended antinociceptive/analgesic effect of the adenosine treatment [
4-
6].
Remifentanil is a short-acting opioid with predictable and rapid recovery that is relatively in dependent of the dose [
7]. A corollary of short action is that patients may experience considerable surgical pain in the immediate postoperative period. Supplemental opioids are thus often given prophyllactically to the patients who are likely to experience postoperative pain. Despite this precaution, postoperative analgesic requirement in patients given intraoperative remifentanil is often surprisingly great [
8]. This observation suggests that remifentanil may be associated with opioid induced hyperalgesia (OIH).
We hypothesized that intraoperative remifentanil administration results in acute OIH that is manifested by shortening of time of first postoperative analgesic requirement, increased postoperative pain and opioid requirement. We therefore tested whether intravenous adenosine administration reduces intraoperative opioid consumption and opioid induced hyperalgesia.
MATERIALS AND METHODS
After obtaining approval from the institutional review board and written informed consent, 90 ASA I-II adult patients undergoing elective tonsillectomy were enrolled in the study. Patients were excluded if they were less than 18 years of age at the time of the surgery or underwent concurrent procedures at the time of tonsillectomy, including patients with known obstructive sleep apnea who required multilevel surgery. Patients were excluded if they had history of asthma, hepatic, renal and endocrine disorders, coronary artery disease, and ingestion of methylxanthine. Additional exclusionary criteria included patients who used illicit drugs, patients who had a known sensitivity to opiate or xanthine agonist or antagonist drugs (e.g. dipyridamole, aminophylline).
Patients were informed that they had equal chances of being randomly (sealed envelope method) assigned into three groups that receive either adenosine (adenosin group) or saline (remifentanil group and sevoflurane group) intravenously. The patients in adenosine group received adenosine at dose of 80 µg/kg/min, and those in remifentanil group and sevoflurane group received an equal volume of saline 10 minutes after the induction of anesthesia until the end of surgery. All patients were premedicated with midazolam 2-3 mg and glycopyrrolate 0.2 mg intramuscularly before arrival in the operating room. Routine monitors included pulse oximeter, automated cuffed blood pressure, electrocardiogram, and end-tidal CO2.
Induction of anesthesia was commenced with a slow (30-60 s) i.v. bolus dose of remifentanil 1 µg/kg and followed by propofol 1 mg/kg in adenosine group and remifentanil group and with propofol 2 mg/kg in sevoflurane group and tracheal intubation was facilitated with rocuronium 0.9 mg/kg in all groups. The anesthesia level was monitored using the BIS method. The BIS electrodes were placed on the forehead and connected to BIS monitoring system (BIS XP, A-2000, Aspect Medical Systems, USA). In adenosine group and remifentanil group, after intubation of trachea, end-tidal sovoflurane concentration was maintained at 1 minimum alveolar concentration (MAC), adjust to age. The patients in sevoflurane group received an equal volume and of saline in place of remifentanil, the concentration of sevoflurane was titrated to maintain a bispectral index in the range 40 to 60. Remifentanil was started at a rate of 0.1 µg/kg/min and subsequently stepwise by 0.05 µg/kg/min increments if inadequate analgesia was suspected. Our criteria for possibly insufficient anesthesia were a heart rate that exceeded preinduction values by 15% and/or a systolic blood pressure that exceed baseline values by 20% at least 5 min.
If the mean arterial pressure decreased during the operation by more than 20% of that recorded before induction of anesthesia, the patients received 10 mg i.v. bolus doses of ephedrine. If the heart rate decreased to less than 50 beats/min, 0.5 mg i.v. atropine bolus was administered. At the end of surgery, neuromuscular blockade was reversed with neostigmine 0.05 mg/kg and atropine 0.02 mg/kg when the train-of-four ratio had returned to 25%. When BIS values reached 80 and spontaneous breathing was achieved, extubation was performed. Remifentanil and adenosine infusion were discontinued when the last surgical stitch was placed.
Pain scores at swallowing saliva or other fluids were documented using the 10-point linear visual analog scale which is a straight line with the left end of the line representing no pain and the right end of the line representing the worst pain. Patients are asked to mark on the line where they think their pain is. Intraoperative evaluation included time weighted mean remifentanil dose, and postoperative evaluation included degree of pain severity at 30 minute, and 6, 12, and 24 hours, the time to first postoperative analgesic (pethidine or ketorolac) requirement, and analgesic dose required during 24 hours. Side effects including postoperative hemorrhage, postoperative oxygen desaturation, readmission for pain, hypotension, bradycardia and transient atrioventricular block were also recorded. A trained anesthesiologist who was not involved in the study assessed pain, and analgesic consumption. An i.v. dose of pethidine 25 mg if patients reported VAS ≥ 4 or i.v. dose of ketorolac 30 mg if VAS < 4 were administered during recovery.
On the basis of a preliminary investigation that showed hypothesized means of time to first postoperative analgesic requirement in postoperative period in adenosine group, sevoflurane group and remifentanil group were 36.12 minute, 40.22 minute and 45.32 minute respectively, and standard deviation of subjects was 9.12. Sample size of 27 patients per group is needed to demonstrate a significant difference, with power of 80% and an α coefficient of 0.05.
The results are presented as mean ± SD or the number of patients. Comparisons of age, body weight, operation time, time weighted mean remifentanil dose, the time to first postoperative analgesic requirement, VAS scores for pain and administered analgesics were conducted using One-way ANOVA with tukey's post-hoc multiple comparisons. Pearson Chi-Square test was used to analyze categorical data such as gender, hypotension, bradycardia and postoperative hemorrhage. Fisher's exact test was used to analyze categorical data such as Transient first degree arioventricular block, postoperative oxygen desaturation, prolonged hospitalization (at least 5 days), and readmission for pain control. P < 0.05 was considered statistically significant.
RESULTS
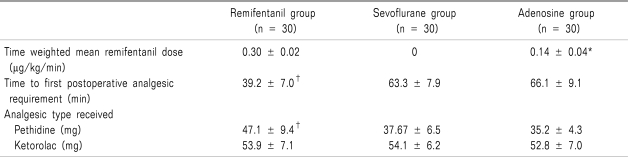
The three groups were comparable with respect to age, gender distribution, weight, and operation time (
Table 1).
Time weighted mean remifentanil dose during intraoperative period in adenosine group was significantly lower than that of remifentnail group (
P = 0.00). The first postoperative analgesic were required earlier in remifentanil group than sevoflurane group or adenosine group (
P = 0.00), but not significant between adenosine group and sevoflurane group. Pethidine but not ketorolac requirement during 24 hours in sevoflurane group and adenosine group was significantly lower than that of remifentnail group (
P = 0.00) (
Table 2).
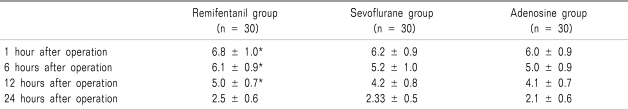
The VAS scores for pain in sevoflurane group and adenosine group were significantly lower than those of remifentnail group for 12 hours after operation (
P = 0.00) (
Table 3).
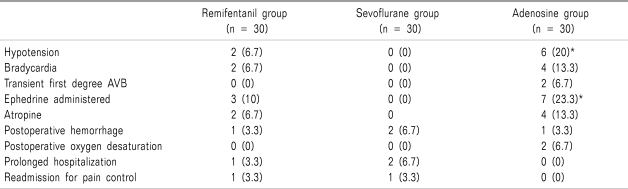
Incidence of hypotension (
P = 0.024) and number of ephedrine administered (
P = 0.011) in adenosine group were significantly higher than those of sevoflurane group (
Table 4).
DISCUSSION
We confirmed our hypothesis that intraoperative remifentanil administration results in acute opioid tolerance and OIH that are manifested by earlier requirement of the first postoperative analgesic, increased postoperative pain and opioid requirement in remifentanil group compared with sevoflurane group. In the adenosine group, the findings of the acute opioid tolerance and OIH such as the earlier requirement of the first postoperative analgesic, increased postoperative pain and opioid requirement was reversed to the sevoflurane group.
Adenosine can exert complex effects on pain transmission, with prominent actions at both peripheral and spinal sites in preclinical models. The nature of the modulation of pain signaling depends very much upon the receptor subtype activated. In the periphery, adenosine A1 receptor activation produces pain suppression, while adenosine A
2 and A
3 receptor activation produces pain enhancement [
1-
3].
By administering of adenosine compounds during surgery, significant and long lasting perioperative pain relief can be achieved via central A
1 receptor-mediated antinociceptive/analgesic actions as well as via peripheral A
2a or A
3 receptor-mediated anti-inflammatory actions. Thus, adenosine compounds have significant potential for alleviating various types of pain [
9,
10].
Previous clinical studies have demonstrated that adenosine infusions reduce perioperative anesthetic requirements and the total amount of analgesics used during the postoperative period in patients undergoing abdominal hysterectomy [
4,
6] and isoflurane requirements in patients in patients undergoing shoulder surgery with general anesthesia [
5]. Adenosine infusion was consistently shown to reduce anesthetic requirements in animal investigation [
11]. The method of drug administration appears to influence the analgesic properties of adenosine in patients who undergo surgical procedures with general anesthesia. The anesthetic technique used during surgery may also affect the efficacy of adenosine [
3].
Intravenous/systemic adenosine may attenuate perioperative pain primarily through its anti-inflammatory actions mediated by peripheral adenosine A
2a or A
3 receptors [
12,
13]. Such an anti-inflammatory effect may reduce the ongoing stimulation of peripheral nociceptors by suppressing inflammation following tissue damage [
14,
15]. This assumption is supported by the result of a study showing that an anti-inflammatory drug, ibuprofen, but not a long-acting selective A
1-adenosine receptor agonist, SDZ WAG994, provided significant postoperative analgesia after third-molar surgery [
16].
OIH is most broadly defined as a state of nociceptive sensitization caused by exposure to opioids. OIH may represent one of many reasons for declining levels of analgesia while receiving opioids or a worsening pain syndrome. Another manifestation might be the experience of excessive pain after an otherwise straightforward surgical procedure. OIH is seen in both humans and in animal models in the settings of very low-dose opioid administration, during maintenance dosing, and when doses are extremely high [
17]. Low-dose OIH effect is mediated through opioid receptors, however high-dose OIH does not seem to be mediated by opioid receptors [
18]. NMDA receptors have been shown to play a key role in in a opioid induced hyperalgesia [
19]. Adenosine A
2a receptors inhibit the conductance of NMDA receptor [
20]. In examining effects of adenosine on tolerance to and dependence upon morphine, somewhat variable results are reported. Thus, some adenosine analogs do not affect, while others decrease, tolerance and dependence.
Postoperative pain scores were also significantly lower during the postoperative period in patients who received adenosine with pronounced and sustained relief lasting up to 48 hours. In a previous study, adenosine-treated patients also required appreciably less opioid analgesics with a reduction of up to 71% in PACU, and up to 45% at 48 hours [
9]. In our study, patients in adenosine group who received intravenous adenosine showed low VAS scores for pain for 12 hours after operation. The effect of adenosine on postoperative pain in our study outlasted the duration of the infusion. It is therefore speculated that adenosine may affect neuronal mechanisms involved in central hyperexcitability [
3,
10,
20] and that such an effect would persist longer than the period of administration of the drug.
Hypotension was the most common adverse event among side effects, and occurred in 20% of adenosine-treated patients versus 6.7% of patients in the remifentanil group. This result suggested drug-to-drug interactions between adenosine, inhaled anesthetics and opioids may worsen the hemodynamic effects of adenosine. The incidence of atrioventricular block was very low in each treatment group.
We conclude that adenosine appears to demonstrate opioid-sparing, and analgesic properties and may be used as an adjunct in the intraoperative periods. Although studies suggest that adenosine might be a useful adjunct analgesic in the perioperative period, more studies are required to confirm these findings. Furthermore, dose-finding clinical studies are warranted to establish the optimal dose for achieving a balance between efficacy and side effects profile for adenosine use in the perioperative setting.
ACKNOWLEDGEMENTS
This Study was Supported by Wonkwang University in 2009.
References
1. Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998; 347:1–11. PMID:
9650842.

2. Sollevi A. Adenosine for pain control. Acta Anaesthesiol Scand Suppl. 1997; 110:135–136. PMID:
9248564.

3. Gan TJ, Habib AS. Adenosine as a non-opioid analgesic in the perioperative setting. Anesth Analg. 2007; 105:487–494. PMID:
17646510.

4. Segerdahl M, Ekblom A, Sandelin K, Wickman M, Sollevi A. Peroperative adenosine infusion reduces the requirements for isoflurane and postoperative analgesics. Anesth Analg. 1995; 80:1145–1149. PMID:
7762842.

5. Segerdahl M, Persson E, Ekblom A, Sollevi A. Peroperative adenosine infusion reduces isoflurane concentrations during general anesthesia for shoulder surgery. Acta Anaesthesiol Scand. 1996; 40:792–797. PMID:
8874564.

6. Segerdahl M, Irestedt L, Sollevi A. Antinociceptive effect of perioperative adenosine infusion in abdominal hysterectomy. Acta Anaesthesiol Scand. 1997; 41:473–479. PMID:
9150774.

7. Thompson JP, Rowbotham DJ. Remifentanil--an opioid for the 21st century. Br J Anaesth. 1996; 76:341–343. PMID:
8785129.

8. Fletcher D, Pinaud M, Scherpereel P, Clyti N, Chauvin M. The efficacy of intravenous 0.15 versus 0.25 mg/kg intraoperative morphine for immediate postoperative analgesia after remifentanil-based anesthesia for major surgery. Anesth Analg. 2000; 90:666–671. PMID:
10702454.

9. Fukunaga AF, Alexander GE, Stark CW. Characterization of the analgesic actions of adenosine: comparison of adenosine and remifentanil infusions in patients undergoing major surgical procedures. Pain. 2003; 101:129–138. PMID:
12507707.

10. Hayashida M, Fukuda K, Fukunaga A. Clinical application of adenosine and ATP for pain control. J Anesth. 2005; 19:225–235. PMID:
16032451.

11. Seitz PA, ter Riet M, Rush W, Merrell WJ. Adenosine decreases the minimum alveolar concentration of halothane in dogs. Anesthesiology. 1990; 73:990–994. PMID:
2240689.

12. Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol. 1994; 76:5–13. PMID:
8175547.

13. Montesinos MC, Desai A, Delano D, Chen JF, Fink JS, Jacobson MA, et al. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum. 2003; 48:240–247. PMID:
12528125.

14. Rane K, Sollevi A, Segerdahl M. Intrathecal adenosine administration in abdominal hysterectomy lacks analgesic effect. Acta Anaesthesiol Scand. 2000; 44:868–872. PMID:
10939701.

15. Rane K, Sollevi A, Segerdahl M. A randomised double-blind evaluation of adenosine as adjunct to sufentanil in spinal labour analgesia. Acta Anaesthesiol Scand. 2003; 47:601–603. PMID:
12699520.

16. Seymour RA, Hawkesford JE, Hill CM, Frame J, Andrews C. The efficacy of a novel adenosine agonist (WAG 994) in postoperative dental pain. Br J Clin Pharmacol. 1999; 47:675–680. PMID:
10383546.

17. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006; 104:570–587. PMID:
16508405.

18. Yaksh TL, Harty GJ, Onofrio BM. High dose of spinal morphine produce a nonopiate receptor-mediated hyperesthesia: clinical and theoretic implications. Anesthesiology. 1986; 64:590–597. PMID:
2938524.

19. Van Elstraete AC, Sitbon P, Mazoit JX, Conti M, Benhamou D. Protective effect of prior administration of magnesium on delayed hyperalgesia induced by fentanyl in rats. Can J Anaesth. 2006; 53:1180–1185. PMID:
17142651.

20. Nörenberg W, Wirkner K, Assmann H, Richter M, Illes P. Adenosine A2A receptors inhibit the conductance of NMDA receptor channels in rat neostriatal neurons. Amino Acids. 1998; 14:33–39. PMID:
9871438.






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download