Abstract
Background
Dense fine speckled (DFS) pattern in antinuclear antibody (ANA) test using indirect immunofluorescence method became to be known recently and it is detected in patients with various chronic inflammatory diseases as well as in healthy individuals. We investigated the relation between DFS pattern and various diseases.
Methods
ANA tests by indirect immunofluorescence method using HEp-2 cell line slide (Kallestad; Bio-Rad, USA) were performed in 2,654 patients for screening of systemic autoimmune diseases. The frequencies of ANA and DFS positivity were analyzed according to sex, age, clinical department and disease.
Results
ANA was positive in 13.3% (352/2,654) of the total patients, and the frequency of DFS pattern was observed in 3.8% (101/2,654) of the total patients and in 28.7% (101/352) of the patients with ANA positivity. Higher frequency of DFS positivity was observed in patients referred from Departments of Rheumatology and Nephrology, but there was no difference in the frequencies of DFS positivity among the patients with ANA positivity. The frequency of DFS pattern was higher in seborrheic dermatitis (14.3%), herpes zoster (11.1%), rheumatoid arthritis (16.9%), systemic lupus erythematosus (15.4%) and Sjogren syndrome (14.3%).
Conclusions
The DFS pattern is a frequent finding (about 28% of ANA positivity) in ANA test using indirect immunofluorescence method. Relatively high frequency of DFS pattern was observed in autoimmune diseases, contrary to the previous observations that DFS pattern is not related with autoimmune diseases. Further studies including the confirmation tests of anti-DFS70 are needed for the identification of relation between DFS pattern and particular diseases.
REFERENCES
1.Ganapathy V., Casiano CA. Autoimmunity to the nuclear auto-antigen DFS70 (LEDGF): what exactly are the autoantibodies trying to tell us? Arthritis Rheum. 2004. 50:684–8.

2.Ochs RL., Muro Y., Si Y., Ge H., Chan EK., Tan EM. Autoantibodies to DFS 70 kd/transcription coactivator p75 in atopic dermatitis and other conditions. J Allergy Clin Immunol. 2000. 105:1211–20.

3.Ge H., Si Y., Roeder RG. Isolation of cDNAs encoding novel transcription coactivators p52 and p75 reveals an alternate regulatory mechanism of transcriptional activation. EMBO J. 1998. 17:6723–9.

4.Singh DP., Ohguro N., Kikuchi T., Sueno T., Reddy VN., Yuge K, et al. Lens epithelium-derived growth factor: effects on growth and survival of lens epithelial cells, keratinocytes, and fibroblasts. Biochem Biophys Res Commun. 2000. 267:373–81.

5.Sugiura K., Muro Y., Nishizawa Y., Okamoto M., Shinohara T., Tomita Y, et al. LEDGF/DFS70, a major autoantigen of atopic dermatitis, is a component of keratohyalin granules. J Invest Dermatol. 2007. 127:75–80.

6.Ochs RL., Stein TW Jr., Peebles CL., Gittes RF., Tan EM. Autoantibodies in interstitial cystitis. J Urol. 1994. 151:587–92.

7.Watanabe A., Kodera M., Sugiura K., Usuda T., Tan EM., Takasaki Y, et al. Anti-DFS70 antibodies in 597 healthy hospital workers. Arthritis Rheum. 2004. 50:892–900.

8.Dellavance A., Viana VS., Leon EP., Bonfa ES., Andrade LE., Leser PG. The clinical spectrum of antinuclear antibodies associated with the nuclear dense fine speckled immunofluorescence pattern. J Rheumatol. 2005. 32:2144–9.
9.Shinohara T., Singh DP., Fatma N. LEDGF, a survival factor, activates stress-related genes. Prog Retin Eye Res. 2002. 21:341–58.

10.Maertens G., Cherepanov P., Pluymers W., Busschots K., De Clercq E., Debyser Z, et al. LEDGF/p75 is essential for nuclear and chromosomal targeting of HIV-1 integrase in human cells. J Biol Chem. 2003. 278:33528–39.

11.Okamoto M., Ogawa Y., Watanabe A., Sugiura K., Shimomura Y., Aoki N, et al. Autoantibodies to DFS70/LEDGF are increased in alopecia areata patients. J Autoimmun. 2004. 23:257–66.

12.Yamada K., Senju S., Shinohara T., Nakatsura T., Murata Y., Ishihara M, et al. Humoral immune response directed against LEDGF in patients with VKH. Immunol Lett. 2001. 78:161–8.

13.Daniels T., Zhang J., Gutierrez I., Elliot ML., Yamada B., Heeb MJ, et al. Antinuclear autoantibodies in prostate cancer: immunity to LEDGF/p75, a survival protein highly expressed in prostate tumors and cleaved during apoptosis. Prostate. 2005. 62:14–26.

14.Muro Y., Sugiura K., Morita Y., Tomita Y. High concomitance of disease marker autoantibodies in anti-DFS70/LEDGF autoantibody-positive patients with autoimmune rheumatic disease. Lupus. 2008. 17:171–6.
Fig. 1.
(A) Dense fine speckled (DFS) pattern of a patient with rheumatoid arthritis in ANA test using indirect immunofluorescense method with HEp-2 cells slides (×400). The features of DFS pattern was characterized by dense, fine speckles in nucleus in interphase and strong staining of chromosome region in mitotic cells in metaphase. (B) Typical speckled pattern with nuclear speckles in interphase cells and negative staining of chromosome region in mitotic cells (×400).
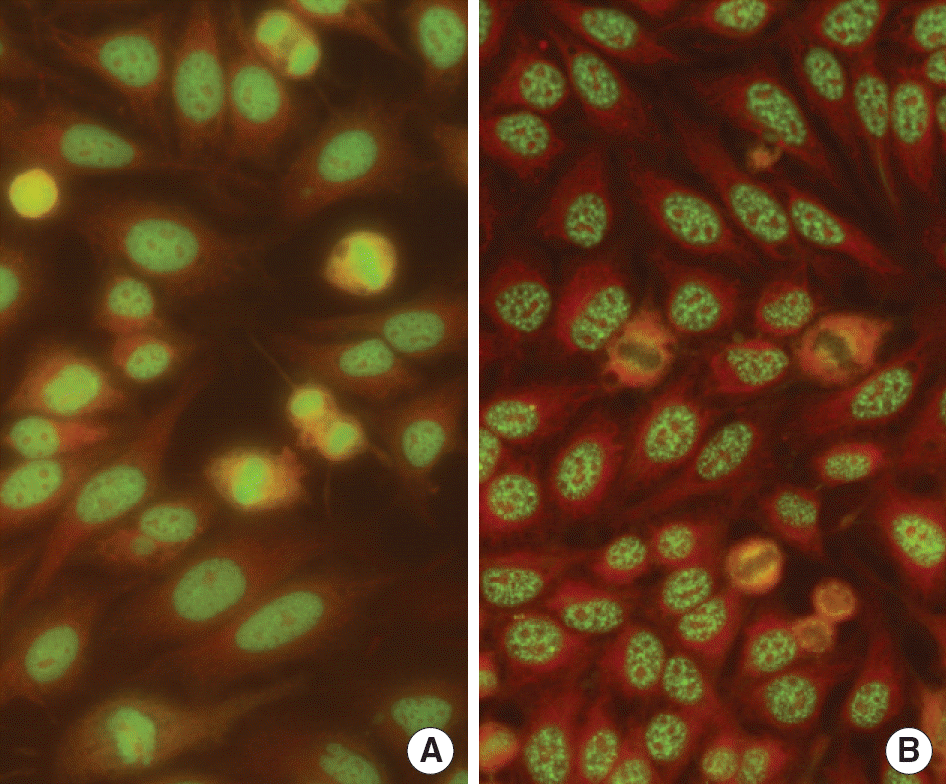
Table 1.
The distribution of age of the patients whose sera displayed positive ANA and dense fine speckled pattern
| Age | No. of patients | ANA positive | DFS positive∗ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Female | Male | Total | Female | Male | Total | Female | Male | |
| 1-10 | 107 | 57 | 50 | 14 (13.1%) | 9 (15.8%) | 5 (10.0%) | 4 (28.6%) | 1 (11.1%) | 3 (60.0%) |
| 11-20 | 258 | 101 | 157 | 31 (12.0%) | 17 (16.8%) | 14 (8.9%) | 14 (45.2%) | 8 (47.1%) | 6 (42.9%) |
| 21-30 | 470 | 222 | 248 | 52 (11.1%) | 35 (15.8%) | 17 (6.9%) | 20 (38.5%) | 13 (37.1%) | 7 (41.2%) |
| 31-40 | 468 | 235 | 233 | 54 (11.5%) | 40 (17.0%) | 14 (6.0%) | 5 (9.3%) | 4 (10.0%) | 1 (7.1%) |
| 41-50 | 538 | 357 | 181 | 74 (13.8%) | 63 (17.6%) | 11 (6.1%) | 20 (27.0%) | 20 (31.7%) | 0 (0.0%) |
| 51-60 | 472 | 303 | 169 | 59 (12.5%) | 50 (16.5%) | 9 (5.3%) | 17 (28.8%) | 14 (28.0%) | 3 (33.3%) |
| 61-70 | 341 | 228 | 113 | 68 (19.9%) | 56 (20.9%) | 12 (4.1%) | 21 (30.9%) | 20 (31.0%) | 1 (8.3%) |
| Total | 2,654 | 1,503 | 1,151 | 352 (13.3%) | 270 (18.0%) | 82 (7.1%) | 101 (28.7%) | 80 (29.6%) | 21 (25.6%) |
| P value† | 0.009 | 0.214 | 0.535 | 0.02‡ | 0.048‡ | 0.016‡ | |||
Table 2.
The distribution of indirect immunofluorescence patterns in 352 ANA positive patients∗
Table 3.
The distribution of clinical department of patients requested ANA test
| Department | No | ANA positivity (%) | DFS pattern (%)∗ |
|---|---|---|---|
| Dermatology | 1,199 | 115 (9.6) | 39 (3.3, 33.9) |
| Rheumatology | 686 | 113 (16.5) | 36 (5.2, 31.6) |
| Nephrology | 302 | 55 (18.2) | 17 (5.6, 30.9) |
| Neurology | 156 | 13 (8.3) | 2 (1.3, 15.4) |
| Others | 311 | 56 (18.0) | 7 (2.3, 12.5) |
| Total | 2,654 | 352 (13.3) | 101 (3.8, 28.7) |
| P value† | <0.01 | 0.015, 0.064‡ |
Table 4.
The clinical diagnosis and frequency of ANA positivity and DFS pattern in patients classified by clinical departments
| Clinical diagnosis | No of patients | No of ANA positivity (%) | No of DFS pattern (%)∗ | ANA titer | |||
|---|---|---|---|---|---|---|---|
| 1:40 | 1:80 | 1:160 | ≥1:320 | ||||
| Dermatology | 1,199 | 115 | 39 | 32 | 7 | ||
| Allergic contact dermatitis | 101 | 23 (22.8) | 8 (7.9, 34.8) | 6 | 2 | ||
| Herpes zoster | 54 | 9 (16.7) | 6 (11.1, 66.7) | 6 | |||
| Atopic dermatitis | 74 | 7 (9.5) | 5 (6.8, 71.4) | 4 | 1 | ||
| Androgenic alopecia | 367 | 22 (6.0) | 5 (1.4, 22.7) | 3 | 2 | ||
| Alopecia areata | 124 | 15 (12.1) | 4 (3.2, 26.7) | 2 | 2 | ||
| Seborrheic dermatitis | 14 | 3 (21.4) | 2 (14.3, 66.7) | 2 | |||
| Urticaria | 113 | 9 (8.0) | 1 (0.9, 11.1) | 1 | |||
| Others | 352 | 27 (7.7) | 8 (2.3, 29.6) | 8 | |||
| P value† | <0.01 | <0.01 0.64 | |||||
| Rheumatology | 686 | 113 | 36 | 10 | 19 | 4 | 3 |
| Rheumatoid arthritis | 65 | 24 (36.9) | 11 (16.9, 45.8) | 4 | 4 | 3 | |
| Ankylosing spondylitis | 87 | 10 (11.5) | 4 (4.6, 40.0) | 4 | |||
| Systemic lupus erythematosus | 13 | 10 (76.9) | 2 (15.4, 20.0) | 2 | |||
| Sjogren syndrome | 7 | 5 (71.4) | 1 (14.3, 20.0) | 1 | |||
| Systemic sclerosis | 2 | 2 (100) | 0 (0, 0) | ||||
| Behcet's disease | 7 | 0 (0) | 0 (0, 0) | ||||
| Raynaud's phenomenon | 4 | 0 (0) | 0 (0, 0) | ||||
| No systemic autoimmune disease | 501 | 62 (12.4) | 18 (3.6, 29.0) | 6 | 8 | 4 | |
| P value † | <0.01 | 0.024 NS | |||||
| Nephrology | 302 | 55 | 17 | 10 | 5 | 2 | |
| Neurology | 156 | 13 | 2 | 1 | 1 | ||
| Others | 311 | 56 | 7 | 4 | 1 | 1 | 1 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download