Abstract
Background
T cell immunophenotypes in patients with hemophagocytic lymphohistiocytosis (HLH) have been described. Downregulation of CD5 or CD7 on T cells has been reported in patients with Epstein-Barr virus (EBV)-positive HLH. As the utility of T cell immunophenotypes as an adjunctive diagnostic or a prognostic marker for HLH has not been evaluated, we analyzed T cell immunophenotypes in HLH patients for this purpose.
Methods
We classified 45 HLH patients into three subgroups: EBV-positive HLH (N=27), EBV-negative secondary HLH (N=15), and familial HLH (N=3). We retrospectively characterized downregulation patterns of CD5 or CD7 on activated T cells, using flow cytometry. Overall survival was estimated using Kaplan-Meier curves and compared using the log-rank test.
Results
An aberrant immunophenotype, including CD5 and/or CD7 downregulation on T cells, was observed in 55.6% (15/27) of the EBV-positive HLH patients and 100% of the familial HLH (3/3). Only one (1/15, 6.7%) patient with EBV-negative secondary HLH showed an aberrant loss of CD7 antigen on CD8+ T cells. The presence of an aberrant T cell immunophenotype was not related to overall survival in EBV-positive HLH and EBV-negative secondary HLH patients.
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening hyperinflammatory syndrome related to excessive immune activation of macrophages and T cells [1]. It is characterized by fever, splenomegaly, cytopenia, hypertriglyceridaemia, hypofibrinogenemia, low or absent natural killer (NK) cell activity, elevated blood levels of the soluble IL-2 receptor and ferritin, and haemophagocytosis in biopsy samples of bone marrow (BM), spleen or lymph nodes [12]. Because these clinical and laboratory findings are often present in various diseases, the diagnosis of HLH may be challenging, especially in early stages of disease. Morphological evaluation of BM aspirates in HLH patients usually shows non-specific abnormal findings such as an increased number of histiocytes and lymphocytes with or without hemophagocytosis and a marked left shift in myelopoiesis [2].
A unique flow cytometric (FCM) finding in Epstein-Barr virus (EBV)-positive HLH patients has been reported: clonal expansion of EBV-infected CD8+ T cells with CD5 or CD7 downregulation [23]. CD5 downregulation on activated CD8+ T cells has also been reported in primary (or familial) HLH patients [4]. Thus, the identification of this clonal T cell population with CD5 or CD7 downregulation might provide important information that would aid in the timely diagnosis of EBV-positive HLH. However, limited data regarding FCM findings for CD8+ T cells are available [23]. Furthermore, the clinical relevance of these cell populations remains unclear; thus, further information regarding T cell abnormalities in EBV-HLH is required. Although T cell immunophenotypes in HLH patients have been described, their utility as an adjunctive diagnostic or a prognostic marker for HLH has not been evaluated. For this purpose, we characterized the downregulation patterns of CD5 or CD7 on activated T cells. Furthermore, we evaluated the laboratory utility of FCM analysis in the initial diagnosis of EBV-HLH by T cell immunophenotyping of BM samples showing hemophagocytosis and examined whether an aberrant T cell immunophenotype in HLH can be used as a prognostic marker.
We retrospectively reviewed medical charts of 60 patients with definite hemophagocytosis in the BM who underwent immunophenotyping of T cells using FCM at Samsung Medical Center, Seoul, Korea, from 2014 to 2017. The follow-up period for patients was between January 2014 and September 2019. The median follow-up duration was 29.8 months (95% confidence interval: 23.3–36.3 months). All patients were evaluated for HLH using the 2004 diagnostic criteria [5]: fever, splenomegaly, cytopenias (affecting at least two of three lineages in the peripheral blood; Hb <90 g/L, platelets <100×109/L, neutrophils <1.0×109/L), hypertriglyceridemia (≥3.0 mmol/L) and/or hypofibrinogenemia (≤1.5 g/L), low or absent NK cell activity, ferritin (≥500 ug/L), soluble IL-2 receptor (≥2,400 U/mL), and a molecular analysis (PRF1 and UNC13D genes). Forty-five patients satisfied five or more of these criteria and were finally diagnosed as having HLH [5]. The remaining 15 were excluded. For an EBV-positive HLH diagnosis, the diagnostic criteria for HLH had to be fulfilled, and EBV infection was determined by either the presence of EBV DNA by viremia (>2,000 copies/mL in serum) or by a positive result for Epstein-Barr encoding region in situ hybridization (EBER-ISH) on a BM section [6].
Patients were categorized into three subgroups. The aforementioned HLH patients with EBV-associated disorders, including EBV infection and EBV-positive lymphoproliferative disorders, were designated as EBV-positive HLH (N=27). HLH patients with other disorders, unrelated to EBV infection, such as autoimmune diseases, infections, and idiopathic cases, were designated as EBV-negative secondary HLH (N=15). More detailed characteristics and laboratory findings of each subgroup are shown in Table 1. HLH patients with genetic mutations were designated as familial HLH (N=3) by detecting pathogenic variants in the UNC13D gene (Table 2). This study was approved by the Institutional Review Board of Samsung Medical Center, Seoul, Korea (SMC 2017-12-095), which waived the need for informed consent.
FCM was performed immediately on fresh BM aspirates in all patients. The samples were collected in EDTA or heparin anticoagulant and routinely processed using a red cell lysis method. Cell suspensions were incubated with combinations of eight monoclonal antibodies (Becton Dickinson, San Jose, CA, USA): CD45-AmCyan, CD3-allophycocyanin-cyanine 7, CD4-pacific blue, CD8-PeCy7, CD56-allophycocyanin, CD2-peridinin chlorophyll protein, CD5-fluorescein isothiocyanate, and CD7-phycoerythrin. Data were acquired using a FACSCanto II flow cytometer (Becton Dickinson) and analyzed using the Kaluza software (Becton Dickinson). CD5 or CD7 downregulation on T cells was determined by adjusting the cutoff range of the non-neoplastic T cell populations reported by Jamal et al. [7]. Three reviewers confirmed the agreement of all analyzed FCM data.
Categorical variables were expressed as frequencies and percentages, and continuous variables were expressed as medians with ranges. The cut-off values of the categorical variables were taken from the 2004 diagnostic criteria for HLH [5].
Survival curves were generated using the Kaplan-Meier method, and survival rates were compared using the log-rank test. Overall survival was measured from the date of diagnosis to the date of all-cause death and was censored at the date of the last follow-up visit. All analyses were performed using SPSS for Windows, version 23 (SPSS Inc., Chicago, IL, USA). P≤0.05 (two-tailed) was considered statistically significant.
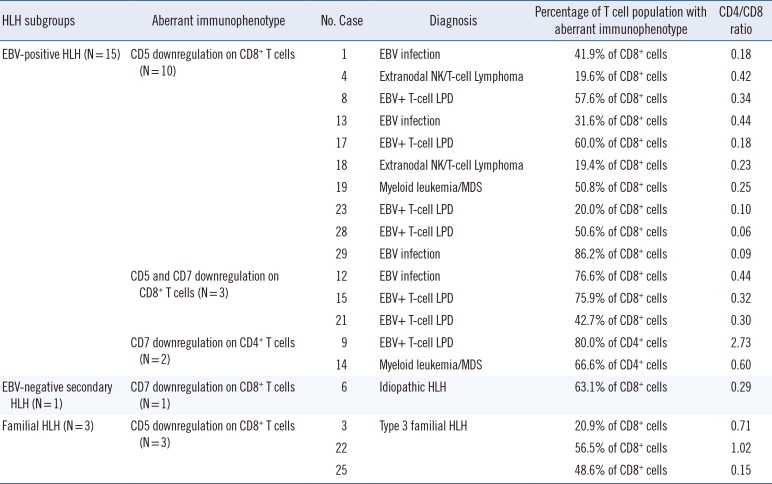
The aberrant immunophenotype patterns observed in the three subgroups are shown in Figs. 1 and 2, and Table 3. Aberrant immunophenotypes, including CD5 and/or CD7 downregulation on CD4+ helper T cells or CD8+ cytotoxic T cells, were observed in 55.6% (15/27) of EBV-positive HLH patients, 6.7% (1/15) of EBV-negative secondary HLH patients, and 100% of familial HLH patients (3/3).
Overall survival did not differ significantly between patients with normal and abnormal immunophenotypes. Subgroup analysis showed that aberrant loss of CD5 or CD7 was not significantly related to overall survival in EBV-positive HLH and EBV-negative secondary HLH patients (Fig. 3).
HLH can be categorized as primary or secondary based on the underlying cause. Primary HLH is associated with a proven genetic etiology or a family history and accounts for the majority of pediatric HLH cases [89]. Familial HLH is caused by mutations in several genes, including PRF1, UNC13D, STX11, and STXBP2. Approximately 70% of familial HLH cases belong to type 2 and type 3 familial HLH, which are caused by PRF1 and UNC13D, respectively [1011]. UNC13D is the predominant causative gene in Korean familial HLH patients [31213]. In our study, all three familial HLH patients were diagnosed as having type 3 familial HLH with a known splicing mutation c.754-1G>C. HLH also occurs secondary to various underlying disorders, including malignancies, rheumatologic diseases, and various infections [1415]. Among infections, EBV is the most common virus triggering the occurrence of HLH [161718]. Therefore, we categorized 45 HLH patients into familial HLH (N=3) and secondary HLH, which was reclassified into EBV-positive HLH (N=27) and EBV-negative HLH (N=15), according to EBV infection status.
EBV infection is usually asymptomatic but occasionally leads to a variety of diseases, such as acute infectious mononucleosis, chronic active EBV infection, EBV-infection-associated HLH, EBV-associated B cell lymphoproliferative disorder (LPD), and EBV-associated T/NK cell LPD [1920]. In the HLH response to EBV, T cells are the major target of EBV infection, leading to polyclonal or monoclonal proliferation of EBV-infected T cells [721]. In infectious mononucleosis, a reactive CD8+ T cell population with dim or absent CD7 is commonly found [22]. Wada et al. [23] reported that a lack of CD5 expression on CD8+ T cells constitutes a unique immunophenotypic feature of EBV-positive HLH. We also identified 15 patients with downregulation of CD5 and/or CD7 expression on T cells in the EBV-positive HLH group, similar to previous reports [2123], and an aberrant T cell immunophenotype was less frequent in EBV-negative HLH than in EBV-positive HLH; 93.3% (14/15) of the EBV-negative secondary HLH patients showed normal T cell immunophenotypes. Our findings extend previous findings that EBV infection is associated with downregulation of CD5 and/or CD7 expression on T cells.
Aberrant T cell immunophenotypes have been reported mostly in patients with EBV-HLH but also in patients with familial HLH [34]. Karandikar et al. [24] revealed expanded subpopulations of CD8+ T cells with the lack of CD5 expression in a patient with familial HLH and showed no evidence of bacterial or viral infection, including cytomegalovirus (CMV) and EBV. Shin et al. [3] also reported a Korean patient with familial HLH with an abnormal population of CD8+ T cells with downregulated levels of CD5 or CD7 and T cell monoclonality and no laboratory evidence of active EBV or CMV infection. Interestingly, aberrant T cell immunophenotypes have been reported in familial HLH patients without evidence of active EBV infection. Wada et al. [4] also found that the activated CD8+ T cells exhibited down-regulation of CD5 and that CD4+ T cells from the patients with FHL2 exhibited normal expression of CD5, although they did not provide any laboratory evidence of active EBV infection. This unique subpopulation is thought to be the result of dysregulated proliferation of CD8+ T cells, similar to clonal proliferation of EBV-infected cells [25]. Consistent with previous reports, we found a lack of CD5 on CD8+ T cells in all three familial HLH patients (as well as one case from reference [3]) without evidence of active EBV infection [426]. These data suggest that the lack of CD5 on CD8+ T cells in familial HLH occurs in various situations other than EBV infection.
Some T cell immunophenotypes can be used to identify clonal T cell disorders, such as skewing of the CD4:CD8 ratio, loss of surface CD4 or CD8, co-expression of CD4 and CD8, and loss of CD5 and/or CD7 [27]. Among them, a lack of CD5 expression on CD8+ T cells could serve as a useful marker of T cell proliferation in EBV-positive HLH. However, EBV infects T cells in EBV-HLH, leading to polyclonal or monoclonal proliferation of EBV-containing T cells, which may mimic T cell LPD (T-LPD) [21]. Thus, careful interpretation is required for the immunophenotyping and clonality data in T cell proliferation in association with EBV-HLH, to avoid erroneous diagnosis of T-LPD. In our study, the aberrant T cell immunophenotype in HLH was helpful in discriminating EBV-negative secondary HLH and EBV-positive HLH, but it may not be useful for discriminating EBV infection in HLH from T-LPDs (data not shown).
The prognosis of EBV-positive HLH may be better than that of familial HLH [282930]. Imashuku et al. [17] performed a longitudinal follow-up study on EBV-positive HLH patients and showed that the majority of effectively treated EBV-positive HLH patients had a good prognosis, except for a few cases of early death. We evaluated whether aberrant T cell immunophenotypes in HLH can be used as a prognostic marker. We did not find a significant association between survival and aberrant loss of CD5 or CD7 expression on T cells in both EBV-positive HLH and EBV-negative secondary HLH patients. However, aberrant loss of CD5 or CD7 expression on T cells tended to confer a survival benefit among EBV-positive HLH patients, although this tendency was not statistically significant. This pattern was repeated among EBV-negative secondary HLH patients. Our findings imply that immunophenotyping in patients with secondary HLH might help proactively discriminate patients showing better outcomes. However, more data are needed to determine the prognostic significance of aberrant T cell immunophenotypes in HLH patients.
In conclusion, our study suggests that the aberrant T cell immunophenotypes in HLH may assist in discriminating EBV-negative secondary HLH and EBV-positive HLH, but they may not be useful as a prognostic marker. Despite the limitation of a relatively small sample size, our study extends previous findings that the aberrant T cell immunophenotypes are more frequent in EBV-positive HLH than in EBV-negative secondary HLH.
References
1. Otrock ZK, Eby CS. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015; 90:220–224. PMID: 25469675.
2. McCall CM, Mudali S, Arceci RJ, Small D, Fuller S, Gocke CD, et al. Flow cytometric findings in hemophagocytic lymphohistiocytosis. Am J Clin Pathol. 2012; 137:786–794. PMID: 22523218.
3. Shin SY, Lee K, Jang MA, Lee ST, Yoo KH, Koo HH, et al. First report on familial hemophagocytic lymphohistiocytosis with an abnormal immunophenotype and T cell monoclonality in Korea. Ann Lab Med. 2015; 35:155–158. PMID: 25553300.
4. Wada T, Sakakibara Y, Nishimura R, Toma T, Ueno Y, Horita S, et al. Down-regulation of CD5 expression on activated CD8+ T cells in familial hemophagocytic lymphohistiocytosis with perforin gene mutations. Hum Immunol. 2013; 74:1579–1585. PMID: 24051121.
5. Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007; 48:124–131. PMID: 16937360.
6. Stevens SJ, Pronk I, Middeldorp JM. Toward standardization of Epstein-Barr virus DNA load monitoring: unfractionated whole blood as preferred clinical specimen. J Clin Microbiol. 2001; 39:1211–1216. PMID: 11283029.
7. Jamal S, Picker LJ, Aquino DB, McKenna RW, Dawson DB, Kroft SH. Immunophenotypic analysis of peripheral T-cell neoplasms. A multiparameter flow cytometric approach. Am J Clin Pathol. 2001; 116:512–526. PMID: 11601136.
8. Brisse E, Wouters CH, Matthys P. Advances in the pathogenesis of primary and secondary haemophagocytic lymphohistiocytosis: differences and similarities. Br J Haematol. 2016; 174:203–217. PMID: 27264204.
9. Ishii E. Hemophagocytic lymphohistiocytosis in children: pathogenesis and treatment. Front Pediatr. 2016; 4:47. PMID: 27242976.
10. Sieni E, Cetica V, Mastrodicasa E, Pende D, Moretta L, Griffiths G, et al. Familial hemophagocytic lymphohistiocytosis: a model for understanding the human machinery of cellular cytotoxicity. Cell Mol Life Sci. 2012; 69:29–40. PMID: 21990010.
11. Sieni E, Cetica V, Hackmann Y, Coniglio ML, Da Ros M, Ciambotti B, et al. Familial hemophagocytic lymphohistiocytosis: when rare diseases shed light on immune system functioning. Front Immunol. 2014; 5:167. PMID: 24795715.
12. Yoon HS, Kim HJ, Yoo KH, Sung KW, Koo HH, Kang HJ, et al. UNC13D is the predominant causative gene with recurrent splicing mutations in Korean patients with familial hemophagocytic lymphohistiocytosis. Haematologica. 2010; 95:622–626. PMID: 20015888.
13. Koh KN, Im HJ, Chung NG, Cho B, Kang HJ, Shin HY, et al. Clinical features, genetics, and outcome of pediatric patients with hemophagocytic lymphohistiocytosis in Korea: report of a nationwide survey from Korea Histiocytosis Working Party. Eur J Haematol. 2015; 94:51–59. PMID: 24935083.
14. Manji F, Wilson E, Mahe E, Gill J, Conly J. Acute HIV infection presenting as hemophagocytic lymphohistiocytosis: case report and review of the literature. BMC Infect Dis. 2017; 17:633. PMID: 28931369.
15. Brisse E, Wouters CH, Andrei G, Matthys P. How viruses contribute to the pathogenesis of hemophagocytic lymphohistiocytosis. Front Immunol. 2017; 8:1102. PMID: 28936212.
16. Marsh RA. Epstein-Barr virus and hemophagocytic lymphohistiocytosis. Front Immunol. 2017; 8:1902. PMID: 29358936.
17. Ishii E, Ohga S, Imashuku S, Yasukawa M, Tsuda H, Miura I, et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007; 86:58–65. PMID: 17675268.
18. Imashuku S. Clinical features and treatment strategies of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Crit Rev Oncol Hematol. 2002; 44:259–272. PMID: 12467966.
19. Kawa K. Epstein-Barr virus--associated diseases in humans. Int J Hematol. 2000; 71:108–117. PMID: 10745621.
20. Dojcinov SD, Fend F, Quintanilla-Martinez L. EBV-positive lymphoproliferations of B- T- and NK-cell derivation in non-immunocompromised hosts. Pathogens. 2018; 7.
21. Lin MT, Chang HM, Huang CJ, Chen WL, Lin CY, Lin CY, et al. Massive expansion of EBV+ monoclonal T cells with CD5 down regulation in EBV-associated haemophagocytic lymphohistiocytosis. J Clin Pathol. 2007; 60:101–103. PMID: 17213357.
22. Weisberger J, Cornfield D, Gorczyca W, Liu Z. Down-regulation of pan-T-cell antigens, particularly CD7, in acute infectious mononucleosis. Am J Clin Pathol. 2003; 120:49–55. PMID: 12866372.
23. Wada T, Kurokawa T, Toma T, Shibata F, Tone Y, Hashida Y, et al. Immunophenotypic analysis of Epstein-Barr virus (EBV)-infected CD8(+) T cells in a patient with EBV-associated hemophagocytic lymphohistiocytosis. Eur J Haematol. 2007; 79:72–75. PMID: 17532761.
24. Karandikar NJ, Kroft SH, Yegappan S, Rogers BB, Aquino VM, Lee KM, et al. Unusual immunophenotype of CD8+ T cells in familial hemophagocytic lymphohistiocytosis. Blood. 2004; 104:2007–2009. PMID: 15205266.
25. Toga A, Wada T, Sakakibara Y, Mase S, Araki R, Tone Y, et al. Clinical significance of cloned expansion and CD5 down-regulation in Epstein-Barr Virus (EBV)-infected CD8+ T lymphocytes in EBV-associated hemophagocytic lymphohistiocytosis. J Infect Dis. 2010; 201:1923–1932. PMID: 20443735.
26. Manno EC, Salfa I, Palma P, Bertaina A, Lombardi A, Moretta F, et al. Familial hemophagocytic lymphohistiocytosis type 3 diagnosed at school age: a case report. J Pediatr Hematol Oncol. 2014; 36:e128–e130. PMID: 23669735.
27. Tembhare P, Yuan CM, Xi L, Morris JC, Liewehr D, Venzon D, et al. Flow cytometric immunophenotypic assessment of T-cell clonality by Vbeta repertoire analysis: detection of T-cell clonality at diagnosis and monitoring of minimal residual disease following therapy. Am J Clin Pathol. 2011; 135:890–900. PMID: 21571962.
28. Mehta RS. Hemophagocytic lymphohistiocytosis (HLH): a review of literature. Medical Oncology. 2013; 30:740. PMID: 24105023.
29. Imashuku S, Teramura T, Tauchi H, Ishida Y, Otoh Y, Sawada M, et al. Longitudinal follow-up of patients with Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Haematologica. 2004; 89:183–188. PMID: 15003893.
30. Ohga S, Kudo K, Ishii E, Honjo S, Morimoto A, Osugi Y, et al. Hematopoietic stem cell transplantation for familial hemophagocytic lymphohistiocytosis and Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in Japan. Pediatr Blood Cancer. 2010; 54:299–306. PMID: 19827139.
Fig. 1
Study design flowchart for classification of HLH patients.
*HLH patients were identified according to the 2004 criteria [13]; †>2,000 genome copies/mL of serum; ‡Three familial HLH cases were diagnosed as type 3 familial HLH based on detection of pathogenic variants of the UNC13D gene.
Abbreviations: HLH, hemophagocytic lymphohistiocytosis; EBV, Epstein-Barr virus; EBER-ISH, Epstein-Barr encoding region in situ hybridization; BM, bone marrow; MDS, myelodysplastic syndromes; LPD, lymphoproliferative disease.
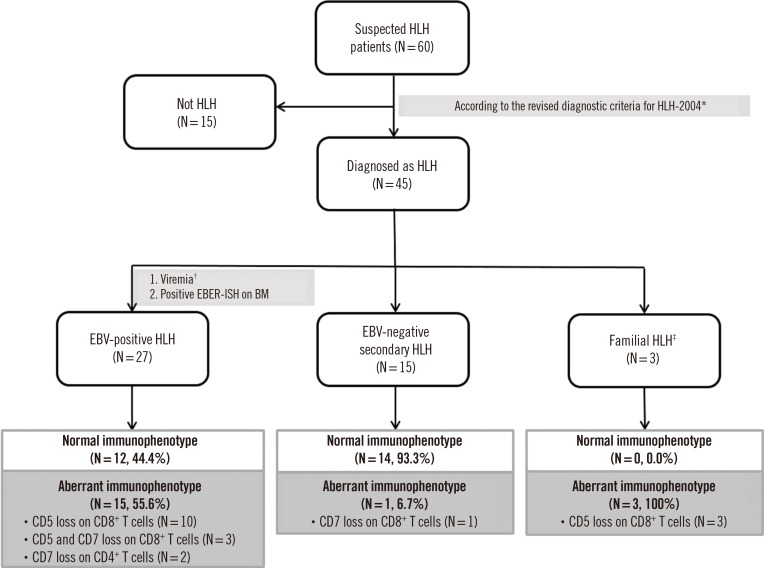
Fig. 2
Representative flow cytometric findings in HLH patients. Red and green colors indicate CD8+ T cells and CD4+ T cells, respectively.
(A) CD5 downregulation on CD8+ T cells in EBV-positive HLH. (B) CD7 downregulation on CD8+ T cells in EBV-negative secondary HLH. (C) CD5 downregulation on CD8+ T cells in familial HLH.
Abbreviations: HLH, hemophagocytic lymphohistiocytosis; EBV, Epstein-Barr virus.
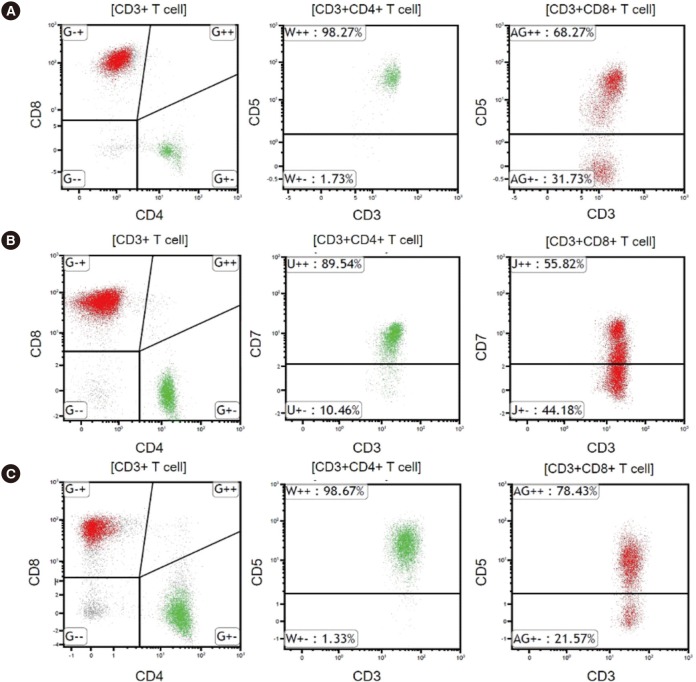
Fig. 3
Kaplan-Meier survival analysis of HLH patients based on the presence of an aberrant T cell immunophenotype. (A) Overall survival of all HLH patients. (B) Overall survival of EBV-positive HLH patients. (C) Overall survival of EBV-negative secondary HLH patients.
Abbreviations: HLH, hemophagocytic lymphohistiocytosis; EBV, Epstein-Barr virus.
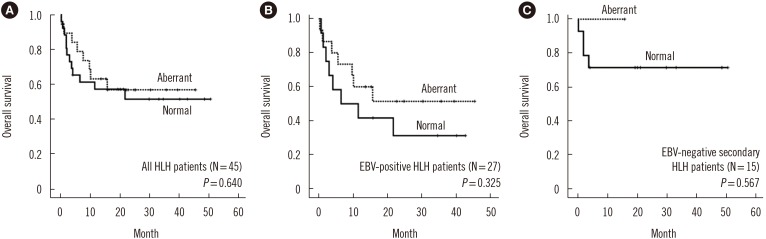
Table 1
Demographics of HLH patients
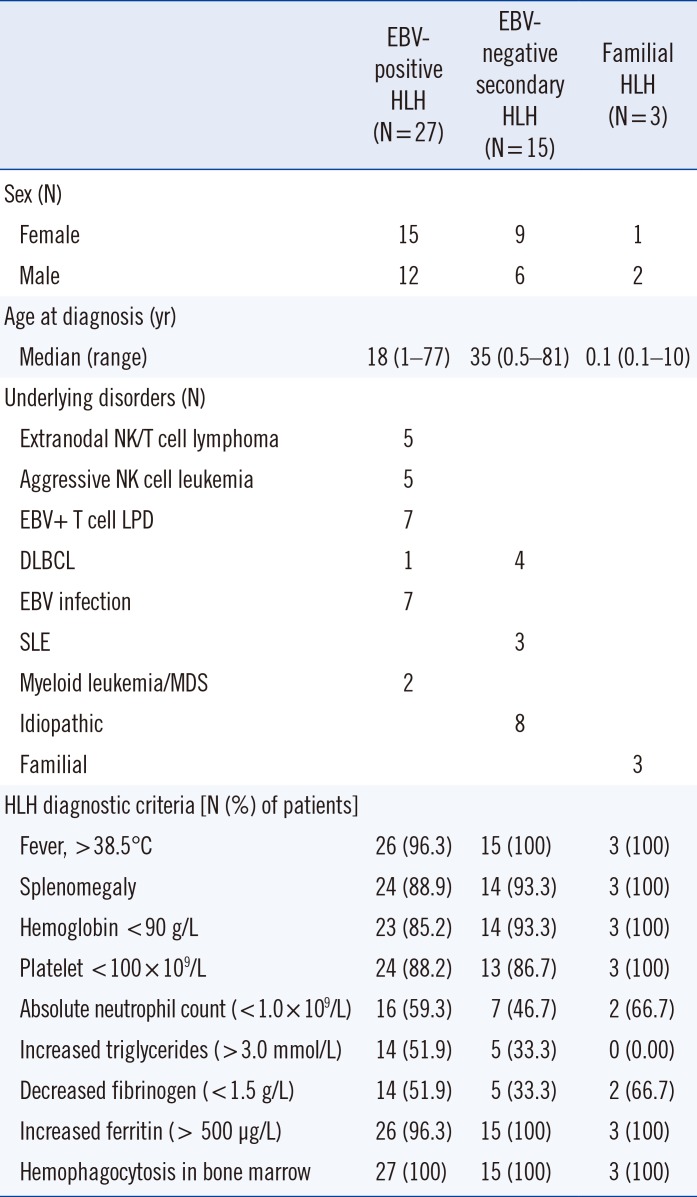
Table 2
UNC13D mutations in three Korean patients diagnosed as having familial hemophagocytic lymphohistiocytosis

| Age at diagnosis | Sex | Mutant allele 1 | Mutant allele 2 | Outcome | Reference |
|---|---|---|---|---|---|
| 10 years | Female | c.544C > T (p.Pro182Ser) | c.754-1G > C | Survived | This study |
| 14 days | Male | c.118-308C > T | c.754-1G > C | Survived | Shin et al.[3] |
| 35 days | Male | c.754-1G > C | c.754-1G > C | Died | This study |
Table 3
Flow cytometric findings for HLH subgroups with an aberrant T cell immunophenotype




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download