Abstract
We investigated the usefulness of age-specific cutoffs for screening of urinary tract infections (UTIs) in Korean outpatients, using the automated urine analyzer UF-1000i (Sysmex, Kobe, Japan). We retrospectively reviewed outpatient medical records. Urine samples of 7,443 outpatients from January 2010 to December 2017 were analyzed using urine culture and UF-1000i. ROC curves were calculated for each UF-1000i parameter based on the culture results. There were 1,398 culture positive samples, 5,774 culture negative samples, and 271 contaminated samples. UF-1000i had an area under the curve of ≥0.9 in outpatients >15 years. The appropriate cutoffs, which are the sum of bacterial (B-A-C) and white blood cell (WBC) counts, were 297.10/µL (15–24 years), 395.65/µL (25–44 years), 135.65/µL (45–64 years), 67.95/µL (65–74 years), and 96.5/µL (≥75 years). B-A-C and WBC counts differed among the three most frequently identified bacteria (Escherichia coli, Klebsiella pneumoniae, and Enterococcus faecalis). The UF-1000i system is useful for applying age-specific cutoffs to screen for UTIs, thereby preventing antibiotic abuse and reducing antibiotic resistance. Future studies can explore how its B-A-C and WBC counts can facilitate selection of empirical antibiotics by distinguishing between gram-positive and gram-negative bacteria.
Urinary tract infections (UTIs) are the most common bacterial infection worldwide, affecting 150 million people annually [1]. Most UTIs occur in outpatients, and outpatients with UTIs (8.6 million each year) account for most of the outpatients in the United States [2]. UTI prevalence varies across age groups [3]; however, its prevalence by age group in Korea is yet to be thoroughly investigated [45].
The diagnostic gold standard for UTIs is urine culture to confirm the presence of bacteria, which takes a minimum of two days. Consequently, empirical antibiotics are typically prescribed for most patients before diagnosis, which increases antibiotic use and resistance [6].
Various alternatives to urine culture, such as dipstick urinalysis, microscopic sediment urinalysis, and automated urinalysis, are used to screen for UTIs. Dipstick urinalysis identifies nitrites and leukocyte esterase in urine. Its sensitivity and specificity were 75% and 82%, respectively, when nitrite or leukocyte esterase positive results are obtained [7]. Microscopic sediment urinalysis aids in UTI diagnosis by identifying bacteria (B-A-C) or white blood cells (WBC). Its sensitivity and specificity were 68.2% and 87.8%, respectively, when pyuria is present during UTI diagnosis [7], and 78.8% and 97.8%, respectively, when confirmed by bacteriuria [8]. Both dipstick urinalysis and microscopic sediment urinalysis are time-consuming and labor-intensive, and their outcomes can be influenced by the technician. Thus, a method of automated urinalysis has emerged [9].
The UF-1000i system (Sysmex, Kobe, Japan) is a type of flow cytometer that combines the impedance method with the identification and quantification of microscopic materials suspended in the urine [10]. UF-1000i has the advantage of having a separate bacterial analysis channel. It can analyze 64,000 particles per sample within 50–75 seconds and analyze 80–100 urine samples per hour. Consequently, it would be appropriate for UTI screening for a high number of urine samples [11]. The default parameters for classifying UTIs, defined as >20 WBCs/µL combined with >50 B-A-C/µL, are not suitable for practical diagnosis. Although some studies have suggested a unified cutoff value for UTI diagnosis for specific ages [12131415], a useful cutoff value is yet to be determined. No studies in Korea have determined a UF-1000i cutoff.
We retrospectively reviewed medical records to evaluate the usefulness of age-specific cutoffs of UF-1000i for rapid screening of UTIs. Our study was approved by the Institutional Review Board of Chung-Ang University Hospital, Seoul, Korea (1802-005-16146). From January 2010 to December 2017, 7,443 urine samples were analyzed. They were obtained from 7,443 outpatients at Chung-Ang University Hospital, aged between 0.5 months and 103 years (4,023 males and 3,420 females). None had received antibiotics within the previous four weeks. Repeat visits of the same outpatients were excluded. Outpatients over 15 years were classified into five age groups (15–24 years, 25–44 years, 45–64 years, 65–74 years, and ≥75 years) on the basis of a previous study on UTI prevalence [3]. Outpatients younger than 15 years were classified into two groups (<4 years and 4–14 years) as samples were collected using a different method for those <4 years.
Samples were collected in a sterile container using the midstream clean catch method except for outpatients <4 years, for whom urine was collected using a sterile pouch [1617]. Samples were analyzed using UF-1000i and urine culture. The UF-1000i analysis was completed within 30 minutes of collection. When 0.8-mL urine samples are loaded on the UF-1000i system along with buffer and two polymethine dyes, the suspended particles in the urine are counted and classified as WBCs, red blood cells (RBCs), epithelial cells, crystals, casts, and B-A-C within one min. Urine culture was performed using a 1-µL loop to streak urine onto blood agar and MacConkey agar plates. The culture medium was inoculated within two hours and was analyzed following incubation at 37℃, 5% CO2 for 24–48 hours. When the culture result indicated that the bacterial load was greater than 105 colony forming units (CFU)/mL, bacterial identification and antimicrobial susceptibility analysis was performed using a Vitek 2 system (BioMérieux Inc., Durham, NC, USA). WBC, B-A-C, and RBC counts from UF-1000i were compared with culture results.
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). Based on the culture results, we generated age-dependent ROC curves and compared them to determine the usefulness of UF-1000i and the cutoffs for each age group. We chose a cutoff with high sensitivity that represents the maximum sum of sensitivity and specificity. The most appropriate sensitivity was ≥90%, the minimum sensitivity was ≥70%, and the minimum specificity was ≥60%. Additional analyses were performed on the three most commonly identified strains in urine culture. The UF-1000i WBC, B-A-C, and RBC counts of each strain were expressed as medians. The Kruskal-Wallis test was used to determine whether they significantly differed among strains, and Mann-Whitney U test was used to compare them between pairs of strains. P<0.05 was considered statistically significant.
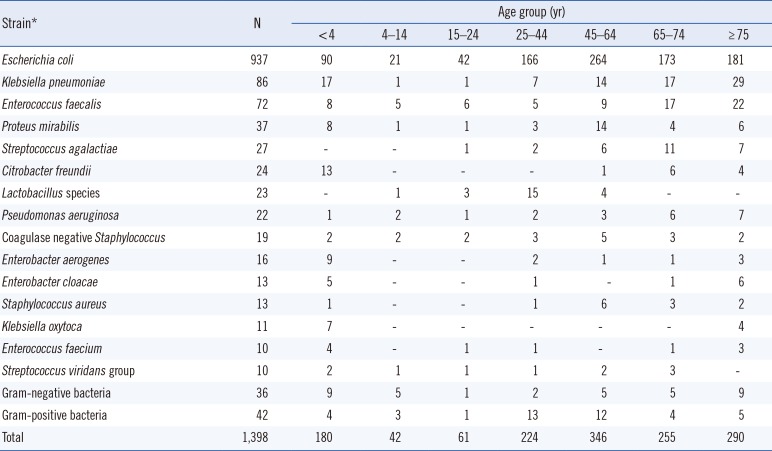
Of the 7,443 samples analyzed, 1,398 were culture positive, 5,774 were culture negative, and 271 were contaminated. Table 1 shows the bacterial species and the number of identified culture positive samples. In all age groups, the area under the curve (AUC) was highest for the sum of the WBC and B-A-C graphs (Fig. 1). The cutoffs of the sums of the WBC and B-A-C graphs in each age group were presented. The AUC for all groups over 15 years of age was >0.9 and with >90% sensitivity.
The three most commonly identified strains in urine culture were Escherichia coli (937 samples), Klebsiella pneumoniae (86 samples), and Enterococcus faecalis (72 samples). E. coli and K. pneumoniae infections resulted in higher B-A-C and WBC counts than E. faecalis infections (Fig. 2). Post-hoc analyses showed the following: E. coli vs E. faecalis (WBC [P<0.001], B-A-C [P<0.001], RBC [P=0.065]), E. faecalis vs K. pneumoniae (WBC [P=0.011], B-A-C [P<0.001], RBC [P=0.818]), and E. coli vs K. pneumoniae (WBC [P=0.577], B-A-C [P=0.752], RBC [P=0.063]).
Our results showed that UF-1000i is suitable for UTI screening in outpatients ≥15 years but not in outpatients <15 years. This may be due to problems associated with collecting urine samples from younger outpatients and the possibility of a high contamination rate. Studies in other countries have not considered UTI prevalence by age [12131415]. However, we showed that applying a single cutoff to all ages is not appropriate for UTI screening because the value varies with age.
Further, if UF-1000i can be used to obtain additional information on bacteria besides screening for UTIs, it may enable the use of empirical antibiotics before confirmatory culture results can be obtained. Monsen et al. [18] compared the B-A-C, WBC, and RBC counts identified by UF-1000i between various uropathogens. They reported high bacterial and WBC counts for E. coli (348 WBCs/µL, 17,914 bacteria/µL) and Klebsiella species (160 WBCs/µL, 24,240 bacteria/µL). In contrast, infection by Enterococci, including E. faecalis, had low bacterial counts (2,103/µL, P<0.05) [18]. Our results were consistent with those of Monsen et al. [18] and may be due to the low measurement of gram-positive bacteria, which are present in clusters or chains. Although there were insufficient samples in our analysis to provide an accurate cutoff for distinguishing between gram-positive and gram-negative bacteria, analysis of additional results in subsequent studies could aid in the selection of antibiotics before the final culture results are available. Due to lack of information on the UTI prevalence in Korea, we divided age groups based on data from other countries. If the age-specific prevalence of UTIs in the Korean population is established, it will be possible to obtain more useful results for this population through age-specific analysis.
In conclusion, it is useful for applying the age-specific cutoffs in UF-1000i for UTI screening, thereby preventing antibiotic abuse and reducing antibiotic resistance. Its B-A-C and WBC counts may facilitate selection of empirical antibiotics by distinguishing between gram-positive and gram-negative bacteria.
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2015R1D1A1-A01058906).
References
1. Stamm WE, Norrby SR. Urinary tract infections: disease panorama and challenges. J Infect Dis. 2001; 183(S1):S1–S4. PMID: 11171002.
2. Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat 13. 2011; 4. 1–38. PMID: 21614897.
3. Schmiemann G, Kniehl E, Gebhardt K, Matejczyk MM, Hummers-Pradier E. The diagnosis of urinary tract infection: a systematic review. Dtsch Arztebl Int. 2010; 107:361–367. PMID: 20539810.
4. Huh JS. The prevalence of urinary tract infections in institutionalized vs. noninstitutionalized elderly persons. Urogenit Tract Infect. 2016; 11:56–61.
5. Lee KY. New insights for febrile urinary tract infection (acute pyelonephritis) in children. Child Kidney Dis. 2016; 20:37–44.
6. Choe HS, Lee SJ, Cho YH, Çek M, Tandoğdu Z, Wagenlehner F, et al. Aspects of urinary tract infections and antimicrobial resistance in hospitalized urology patients in Asia: 10-year results of the Global Prevalence study of Infections in Urology (GPIU). J Infect Chemother. 2018; 24:278–283. PMID: 29292177.
7. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002; 287:2701–2710. PMID: 12020306.
8. Kayalp D, Dogan K, Ceylan G, Senes M, Yucel D. Can routine automated urinalysis reduce culture requests? Clin Biochem. 2013; 46:1285–1289. PMID: 23810583.
9. Oyaert M, Delanghe J. Progress in automated urinalysis. Ann Lab Med. 2019; 39:15–22. PMID: 30215225.
10. Laiwejpithaya S, Wongkrajang P, Reesukumal K, Bucha C, Meepanya S, Pattanavin C, et al. UriSed 3 and UX-2000 automated urine sediment analyzers vs manual microscopic method: a comparative performance analysis. J Clin Lab Anal. 2018; 32:e22249.
11. van der Zwet WC, Hessels J, Canbolat F, Deckers MM. Evaluation of the Sysmex UF-1000i® urine flow cytometer in the diagnostic work-up of suspected urinary tract infection in a Dutch general hospital. Clin Chem Lab Med. 2010; 48:1765–1771. PMID: 20726812.
12. Hu X, Zhang J, Zhang X. Evaluation of the Sysmex UF-1000i urine analyzer as a screening test to reduce the need for urine cultures for urinary tract infection. Lab Med. 2010; 41:349–352.
13. Íñigo M, Coello A, Fernández-Rivas G, Carrasco M, Marcó C, Fernández A, et al. Evaluation of the SediMax automated microscopy sediment analyzer and the Sysmex UF-1000i flow cytometer as screening tools to rule out negative urinary tract infections. Clin Chim Acta. 2016; 456:31–35. PMID: 26921459.
14. Martín-Gutiérrez G, Porras-González A, Martín-Pérez C, Lepe JA, Aznar J. Evaluation and optimization of the Sysmex UF1000i system for the screening of urinary tract infection in primary health care elderly patients. Enferm Infecc Microbiol Clin. 2015; 33:320–323. PMID: 25444045.
15. Pieretti B, Brunati P, Pini B, Colzani C, Congedo P, Rocchi M, et al. Diagnosis of bacteriuria and leukocyturia by automated flow cytometry compared with urine culture. J Clin Microbiol. 2010; 48:3990–3996. PMID: 20739491.
16. Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management. Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011; 128:595–610. PMID: 21873693.
17. LaRocco MT, Franek J, Leibach EK, Weissfeld AS, Kraft CS, Sautter RL, et al. Effectiveness of pre-analytic practices on contamination and diagnostic accuracy of urine cultures: a laboratory medicine best practices systematic review and meta-analysis. Clin Microbiol Rev. 2016; 29:105–147. PMID: 26598386.
18. Monsen T, Rydín P. Flow cytometry analysis using Sysmex UF-1000i classifies uropathogens based on bacterial, leukocyte, and erythrocyte counts in urine specimens among patients with urinary tract infections. J Clin Microbiol. 2015; 53:539–545. PMID: 25472486.
Fig. 1
UF-1000i (Sysmex, Kobe, Japan) ROC curves by age. The sum of B-A-C and WBC graphs showed the highest AUC, so the cutoff of this graph is the most suitable for screening. The optimal ROC curve values (AUC, cutoff, sensitivity and specificity) for each age group were as follows: <4 years (0.790, 74.75/µL, 77.2% and 65.7%), 4–14 years (0.759, 42.9/µL, 81.0% and 65.1%), 15–24 years (0.908, 297.10/µL, 95.1% and 71.7%), 25–44 years (0.902, 395.65/µL, 90.2% and 74.9%), 45–64 years (0.908, 135.65/µL, 90.2% and 72%), 65–74 years (0.916, 67.95/µL, 94.1% and 67.8%), and ≥75 years (0.912, 96.5/µL, 92.8% and 71.1%).
Abbreviations: WBC, white blood cell; B-A-C, bacteria; AUC, area under the curve.
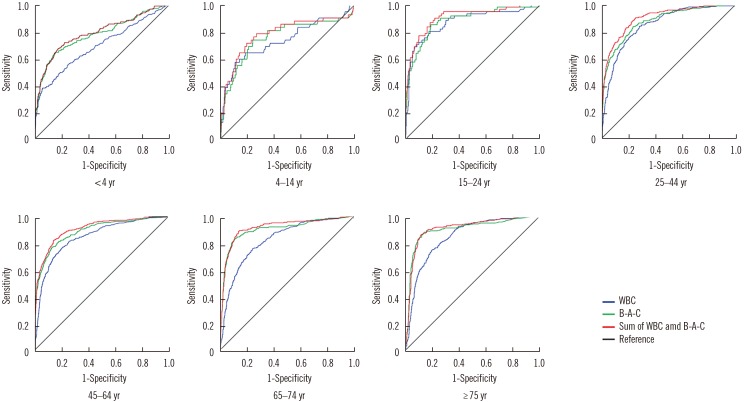
Fig. 2
Graph of median WBC vs B-A-C counts determined by UF-1000i (Sysmex, Kobe, Japan) in outpatients with a urinary tract infection confirmed to be caused by Escherichia coli (937 samples), Klebsiella pneumoniae (86 samples), and Enterococcus faecalis (72 samples). The distribution range (95% confidence interval, minimum, maximum, interquartile range) is as follows: E. coli B-A-C (11,765.0–14,186.4/µL, 2.0/µL, 99,086.0/µL, 17,826.1/µL), E. coli WBC (1,073.3–1,464.4/µL, 0.0/µL, 32,723.0/µL, 1,007.4/µL); E. faecalis B-A-C (1,836.6–5,964.5/µL, 0.0/µL, 53,597.0/µL, 3,016.4/µL), E. faecalis WBC (285.4–847.1/µL, 1.0/µL, 6,779.0/µL, 332.6/µL); K. pneumoniae B-A-C (10,532.2–18,205.2/µL, 2.0/µL, 80,533.0/µL, 20,834.0/µL), and K. pneumoniae WBC (582.3–2,037.0/µL, 1.0/µL, 31,392.0/µL, 1,081.3/µL).
Abbreviations: WBC, white blood cell; B-A-C, bacteria.
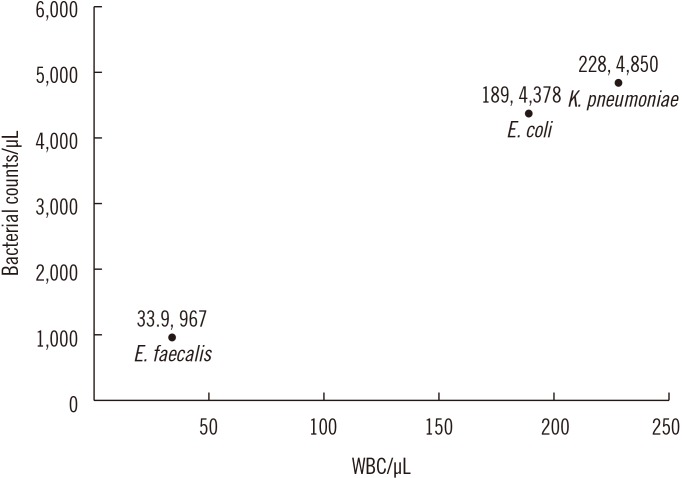
Table 1
Strains identified in urine cultures




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download