Abstract
Background
The increasing prevalence of drug-resistant tuberculosis (TB) infection represents a global public health emergency. We evaluated the usefulness of a newly developed multiplexed, bead-based bioassay (Quantamatrix Multiplexed Assay Platform [QMAP], QuantaMatrix, Seoul, Korea) to rapidly identify the Mycobacterium tuberculosis complex (MTBC) and detect rifampicin (RIF) and isoniazid (INH) resistance-associated mutations.
Methods
A total of 200 clinical isolates from respiratory samples were used. Phenotypic anti-TB drug susceptibility testing (DST) results were compared with those of the QMAP system, reverse blot hybridization (REBA) MTB-MDR assay, and gene sequencing analysis.
Results
Compared with the phenotypic DST results, the sensitivity and specificity of the QMAP system were 96.4% (106/110; 95% confidence interval [CI] 0.9072–0.9888) and 80.0% (72/90; 95% CI 0.7052–0.8705), respectively, for RIF resistance and 75.0% (108/144; 95% CI 0.6731–0.8139) and 96.4% (54/56; 95% CI 0.8718–0.9972), respectively, for INH resistance. The agreement rates between the QMAP system and REBA MTB-MDR assay for RIF and INH resistance detection were 97.6% (121/124; 95% CI 0.9282–0.9949) and 99.1% (109/110; 95% CI 0.9453–1.0000), respectively. Comparison between the QMAP system and gene sequencing analysis showed an overall agreement of 100% for RIF resistance (110/110; 95% CI 0.9711–1.0000) and INH resistance (124/124; 95% CI 0.9743–1.0000).
Tuberculosis (TB) is associated with significant morbidity and mortality and remains one of the deadliest diseases worldwide [1]. Multidrug-resistant tuberculosis (MDR-TB) caused by Mycobacterium tuberculosis (MTB) strains resistant to rifampicin (RIF) and isoniazid (INH) poses a formidable challenge in controlling TB. Therefore, development of more rapid and accurate assays for detecting drug resistance in MTB, especially against first line anti-TB drugs, including RIF and INH, is crucial for effective therapy.
Phenotypic drug susceptibility testing (DST) is the current gold standard for assessing drug resistance in TB. Phenotypic DST of MTB based on microbial growth on solid (Löwenstein–Jensen [LJ] or Ogawa) and liquid media (Mycobacteria growth indicator tube [MGIT]) containing a specified concentration of a single drug is an inexpensive procedure; however, these methods require additional time for completion (approximately 14 days for MGIT and 28 days for LJ and Ogawa) following the primary culture, owing to the slow growth of MTB [234]. Thus, new molecular genotypic methods represent a potentially rapid and sensitive alternative to phenotypic DST. Several commercial diagnostic kits based on nucleic acid amplification are currently available. For example, GeneXpert (Cepheid, Sunnyvale, CA, USA) is a real-time PCR method that directly uses sputum to detect MTB and mutations conferring RIF resistance. Line probe assays based on reverse blot hybridization probes, such as the INNO-LiPA Rif. TB (Fujirebio Europe, Ghent, Belgium) and the GenoType MTBDRplus (Hain Lifescience, Nehren, Germany) assays, are used to rapidly diagnose MDR-TB [567891011].
Recently, a new diagnostic system using a multiplexed, bead-based bioassay with shape-encoded silica microparticles was developed and commercialized [12]. This system, known as Quantamatrix Multiplexed Assay Platform (QMAP; QuantaMatrix, Seoul, Korea), uses encoded microdisk technology; one disk has a 50-µm-thick silica-coated surface and a graphical bar-coded, carboxyl-functionalized magnetic disk that allows > 1,000-plex coding capacity in high-throughput analysis [12]. We developed a QMAP dual-ID (QuantaMatrix), which includes probes to rapidly identify the MTB complex (MTBC) and accurately detect RIF- and INH-resistance-associated mutations (RIF: rpoB, INH: katG, inhA, and ahpC). We evaluated the usefulness of the QMAP system for rapidly identifying MTBC (for four first-line anti-TB drugs: RIF, INH, ethambutol, and pyrazinamide) and for detecting RIF- and INH resistance-associated mutations, using 200 clinical isolates.
Two hundred clinical isolates were prospectively collected from TB patients at the International Tuberculosis Research Center (Changwon, Korea), from June to August 2016 (ClinicalTrials.gov identification number, NCT00341601). Respiratory samples after the decontamination procedure were routinely cultured in Ogawa medium at 37℃. Phenotypic DST for first- and second-line drugs was performed using the absolute concentration method using LJ based M-KIT plates (Korean Institute of Tuberculosis, Osong, Korea). This study was approved by the Institutional Ethics Committee of Yonsei University Wonju Severance Christian Hospital (approval no. CR316304).
One colony (0.2 µm diameter) of each clinical Mycobacterium isolate was collected using a sterile inoculation loop, and the prepared cell suspension was centrifuged for 10 minutes at 13,000×g. Each cell sample was extracted using DNA extraction solution (5% Chelex resin, BioRad, USA) prior to boiling for 10 minutes. The isolated genomic DNA was stored at −20℃ and was used for PCR amplification after complete thawing.
The QMAP system was used according to the manufacturer's instructions [13]. Each sample was tested in duplicate, and all QMAP test runs were performed twice. The MTB-specific oligonucleotide probe was designed to distinguish MTB from nontuberculous mycobacteria (NTM) based on the 16S-23S rRNA internal transcribed spacer region of Mycobacterium species found in the National Center for Biotechnology Information (NCBI) database. Ten RIF probes and 11 INH probes were used. Five of the 10 RIF probes are probes for the wild-type (WT) rpoB region encoding amino acids 509–534. We also used five mutant-type (MT) RIF probes that account for the most common specific mutations [MT1, 513CAA(Gln)-CCA(Pro); MT2, 516GAC(Asp)-TAC(Tyr); MT3, 516GAC(Asp)-GTC(Val); MT4, 526CAC(His)-TAC(Tyr); and MT5, 531TCG(Ser)-TTG(Leu)]. The 11 INH probes included two katG-, four inhA-, and five aphC-probes. Each katG-WT and katG-MT probe detects mutations at katG codon315. The two inhA-WT probes and two inhA-MT probes check for mutations at positions −15 and −8 of the mabA-inhA promoter region. The five ahpC-WT probes check for mutations from positions −66 to +44 of the intergenic region of oxyR-ahpC.
Each of the target Mycobacterium species genes was complementary to a single probe, which was coupled with 5′-amine-modified carboxylated microdisks. Briefly, PCR was performed using a 20 µL reaction mixture (GeNet Bio, Daejeon, Korea) containing 2× master mix, 1× biotinylated primer mixture, 5 µL of sample DNA, and ddH2O up to a final volume of 20 µL. The 40 PCR cycles consisted of initial denaturation at 95℃ for 30 seconds, followed by annealing and extension at 65℃ for 30 seconds; following the final cycle, the samples were maintained at 72℃ for 10 minutes to complete the synthesis of all strands. The biotinylated PCR products were denatured at 25℃ for 5 minutes in a denaturation solution, diluted in 45 µL of hybridization solution, and added to the coupled microdisks in the provided glass MatriPlate (Brooks, Chelmsford, MA, USA). Denatured single-stranded PCR products were hybridized with the coupled probes on the microdisks for 30 minutes at 40℃. The microdisks were then washed three times with gentle shaking in 120 µL of washing solution (0.1% SDS/PBS buffer, pH 7.5) for one minute at 25℃, incubated with 1:2,000 diluted streptavidin R–phycoerythrin conjugate (ProZyme, San Leandro, CA, USA) in conjugate diluent solution for 10 minutes at 25℃, and washed three times with 120 µL of washing solution for one minute at 25℃. Finally, bright-field and fluorescence microscopy images were obtained for data analysis. The fluorescence intensity of all microdisks in the image was automatically measured using the provided software (QMAP V2, QuantaMatrix). The cut-off value for distinguishing between positive and negative results was a fluorescence intensity signal of 500 [13].
The REBA MTB-MDR assay (YD Diagnostics, Yongin, Korea) was performed according to the manufacturer's instructions. Each sample was tested in duplicate. Briefly, PCR was performed using a 20 µL reaction mixture (GeNet Bio) containing 2× master mix, 1× biotinylated primer mixture, 5 µL of sample DNA, and ddH2O up the final volume of 20 µL. The 40 PCR cycles consisted of initial denaturation at 95℃ for 30 seconds followed by annealing and extension at 65℃ for 30 seconds; following the final cycle, samples were maintained at 72℃ for 10 minutes. The amplified target was visualized as a single band corresponding to a size of 150 bp, using the Chemi Doc system (Vilber Lourmat, Eberhardzell, Germany). The PCR products were subjected to REBA. Hybridization and washing were performed according to the manufacturer's instructions. Briefly, biotinylated PCR products were denatured in denaturation solution at 25℃ for five minutes, diluted in 970 µL of hybridization solution, and added to the REBA membrane strip in the provided blotting tray. Denatured single-stranded PCR products were hybridized with the probes on the strip at 50℃ for 30 minutes. The strips were then washed twice with gentle shaking in 1.0 mL of washing solution for 10 minutes at 63℃, and incubated at 25℃ with 1:2,000 diluted streptavidin-alkaline phosphatase conjugate (Roche Diagnostics, Mannheim, Germany) in conjugate dilution solution (CDS) for 30 minutes. Following incubation, the strips were washed twice with 1.0 mL of CDS at 25℃ for one minute. The colorimetric hybridization signals were visualized by adding 1:50 diluted alkaline phosphatase-mediated staining solution and nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP; Roche Diagnostics) and incubating the strips until the color was detected. Finally, the band pattern was read and interpreted [13].
The corresponding resistance-associated mutations were sequenced with designed specific primers: rpoB F (5′-GCGTCGGTCGCTATAAGGT-3′)/R (5′-ACGGGTGCACGTCGCG-3′); katG F (5′-CACACTTTCGGTAAGACCCA-3′)/R (5′-GAAACTGTTGTCCCATTTCG-3′); promoter of mabA-inhA F (5′-CGCTGCCCAGAAAGGGA-3′)/R (5′-TCCTCCGGTAACCAGGACTC-3′); and intergenic region of oxyR-ahpC F (5′-ACTGCTGAACCACTGCTTTGC-3′)/R (5′-TGATCGCCAATGGTTAGCAG-3′). The amplified DNA products were sequenced using the ABI Prism BigDye Terminator Kit on an ABI 3730 automated DNA sequencer (Cosmo Genetech, Seoul, Korea) and compared with sequences in the NCBI GenBank database.
All statistical analyses were performed using SPSS version 23.0 (IBM, Armonk, NY, USA). Sensitivity, specificity, and positive and negative predictive values, with 95% confidence intervals (CIs), were estimated. Detection of RIF and INH susceptibility by the QMAP system and REBA MTB-MDR assay was analyzed using the kappa coefficient: values of 0.75 and higher indicate especially good agreement.
Of the 200 MTBC isolates, 46 (23.0%) were pan-susceptible to all four first-line anti-TB drugs (RIF, INH, ethambutol, and pyrazinamide). Of the 154 (77.0%) anti-TB drug-resistant isolates, 110 (71.4%) were RIF-resistant (only 10 RIF mono-resistant), and 144 (93.5%) were INH-resistant (44 INH mono-resistant). Of the 100 (64.9%) MDR-TB (RIF-resistant and INH-resistant) isolates, 18 (18.0%) were resistant to ethambutol and pyrazinamide.
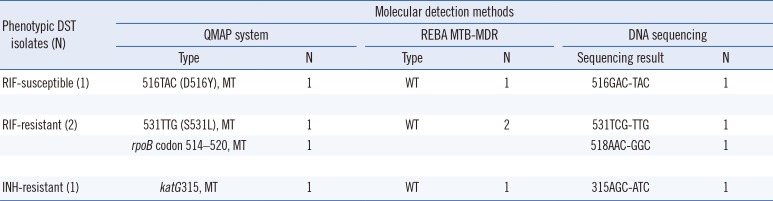
The phenotypic DST and QMAP system results for 22 of the 200 MTBC isolates were inconsistent: 18 isolates were RIF-susceptible as per phenotypic DST, but had mutations as detected by QMAP, and four isolates determined to be RIF-resistant by phenotypic DST did not have any mutations as per the QMAP system. The overall agreement between phenotypic DST and the QMAP system was 89% (N=178; 95% CI: 0.8385–0.9268). The most frequently mutated rpoB codons were codons 531 [48.8% (62/127)], 524–529 [11.8% (15/127)], 530–534 [11.8% (15/127)], 516 [9.4% (12/127)], 514–520 [7.1% (9/127)], and 526 [5.5% (7/127)]. Mutation analysis of the RIF resistance-determining region (RRDR) for these 22 discrepant isolates revealed a 100% agreement rate between the QMAP system and DNA sequence analysis (Table 1).
The QMAP system demonstrated that 75.0% (108/144, 95% CI 0.6731–0.8139) of the phenotypically INH-resistant strains and 3.6% (2/56) of the phenotypically INH-susceptible strains had mutations or were missing WT probes in one or more of the three INH regions (katG, inhA, and ahpC). The most frequent mutation site was katG315 AGC-ACC (68.5%, 76/111), while 21.6% (24/111) of the INH-resistant strains harbored mutations in the promoter region of mabA-inhA. Most of the mutations were observed at position -15 (C-15T; 95.8%, 23/24). Two strains susceptible to INH by phenotypic DST were identified as INH-resistant by QMAP, as there were losses in katG and ahpC WT bands. Sequence analysis confirmed the mutations in katG codon 315 and the ahpC promoter region. Additionally, 36 (25.0%) INH-resistant isolates by phenotypic DST were susceptible to INH as per the QMAP system and sequence analysis (Table 2).
Compared with phenotypic DST, the RIF resistance detection sensitivity and specificity of the QMAP system were 96.4% (106/110) and 80.0% (72/90), respectively. Compared with phenotypic DST, the INH resistance detection sensitivity and specificity of the QMAP system were 75.0% (108/144) and 96.4% (54/56), respectively. Compared with those of rpoB sequence analysis, the RIF resistance detection sensitivity and specificity of the QMAP system were 100% (95% CI: 0.9743–1.0000; P<0.0001) and 100% (95% CI: 0.9587–1.0000; P<0.0001), respectively (Table 3).
The probes used in the QMAP system and REBA MTB-MDR assay to detect mutations related to drug resistance bind the same site; however, there are some differences in the other nucleotide sequences (Table 4). The agreement rates between the REBA MTB-MDR assay and the QMAP system for RIF and INH resistance detection were 98.5% (197/200; 95% CI: 0.9548–0.9969; kappa=0.968) and 99.5% (199/200; 95% CI: 0.9694–1.000; kappa=0.990), respectively (Table 5). For RIF results of 200 isolates, there were three discrepant isolates, which were MT [516TAC, 531TTG, 514–520] according to the QMAP system but WT according to the REBA MTB-MDR assay. rpoB sequence analysis of these isolates revealed 531TTG, 516TAC, and 518GGC, indicating that the QMAP system correctly detected the mutations. For INH results of 200 isolates, one discrepant case, in which a katG 315 MT was detected by the QMAP system and katG 315 WT by the REBA MTB-MDR assay, was found to harbor katG 315 AGC-ATC by sequence analysis. Thus, the different sets of probes used by QMAP and REBA can cause discrepancy in the data (Table 6).
The QMAP system allows for the simultaneous discrimination of MTB and detection of RIF and INH resistance-associated mutations. This system can analyze nucleic acids as well as proteins, and is rapid and relatively easy to perform in terms of equipment operation and software. It is based on a combination of multiplex PCR followed by hybridization of the amplicons to a microdisk with immobilized probes covering WTs and MTs. In the QMAP system, 12 fields (the area in which one microwell is divided; x-axis 3×y-axis 4) of the microwell are captured and the average fluorescence intensity is calculated. Thus, this system, which provides equal or superior accuracy relative to conventional phenotypic methods, can readily differentiate bacterial species that have similar phenotypic characteristics and does not involve subjective interpretation [14].
RIF resistance is caused by mutations in the β-subunit of RNA polymerase, a target of RIF encoded by the rpoB gene [4]. Over 95% of RIF-resistant isolates harbor mutations within the 81-bp hot-spot region (RRDR, codons 507–533) of the rpoB gene [15], with codons 516, 526, and 531 [16] or codons 531, 526, 516, 533, and 513 [17] being the most frequent mutations. Moreover, mutations at codon 315 of katG, positions −15 and −8 of the mabA-inhA promoter, and positions −6 to −47 of the oxyR-ahpC intergenic region are responsible for 80–90% of INH-resistant strains [18]. In our study, 96.0% (119/124) of the RIF-resistant isolates and 75.3% (110/146) of the INH-resistant isolates had mutations in the RRDR and INH resistance-conferring region, respectively. In regard to RIF resistance detection, the QMAP system showed sensitivity and specificity similar to previous studies; 93.5–98% and 99% for the GenoType MTBDRplus assay (Hain Lifescience) [1920], 97% and 100% for the Anyplex assay (Seegene, Seoul, Korea) [21], 92% and 74% for the LightCycler platform (Roche Diagnostics) [22], and 100% and 99.4% for the BluePoint MycoID plus kit (Bio Concept Corporation, Taichung, Taiwan) [23], respectively. The specificity of the QMAP system for RIF resistance was 80.0% compared with DST. There may be several causes for such low specificity. We hypothesized that the main cause is sample selection bias. INH resistance detection using the QMAP system was similar (75–93.9% sensitivity and 99% specificity) to that using the MTBDRplus assay [1920], while the Anyplex assay exhibited 61% sensitivity and 98% specificity for INH [21]. Consequently, as only the mutations included in the QMAP system probes could be detected, some resistant phenotype isolates without QMAP-detected mutations may have harbored mutations at other loci.
RRDR sequence analysis of the 18 isolates showing discrepancy between phenotypic RIF DST and the QMAP system identified mutations not included in the QMAP system probes (P511L, G513L, M515V, H526A, H526L, H526N, H526C, S531P, and A532V). When phenotypic DST for INH was regarded as the standard method, the QMAP system showed low sensitivity (75.0%) for INH resistance detection. This was most likely due to mutations in other codons of katG, inhA, and ahpC or drug-resistant loci. These results are similar to those in a study showing 10–25% low level INH-resistant strains with no known resistance mutations [24]. Clearly, additional studies will be necessary to address undefined genes and the many underlying mechanisms in these cases. Furthermore, we found 98.5% and 99.5% agreement between the QMAP system and REBA MDR-MTB assay. Among the isolates that showed discrepant results between these two molecular DST methods, the QMAP system showed higher agreement with the sequence analysis of the RRDR or INH-associated region than with the REBA MDR-MTB assay. The discrepant results may have been due to technical error or assay accuracy. In order to exclude the possibility of technical error, we repeated the experiments twice; however, we obtained the same results. In addition, we analyzed the discrepant results by comparing DNA sequencing results and the results of two molecular methods. Based on this analysis, we conclude that differences in accuracy between the QMAP system and REBA assay can lead to discrepant results.
There were several limitations in our study. Owing to a lack of patient clinical history, we could not correlate the prevalence of drug resistance in newer or previously treated cases and other probable reasons for MDR predisposition. Moreover, the QMAP system was evaluated using only clinical isolates. Therefore, further studies are necessary to examine the impact of direct detection of drug resistance in respiratory samples. Validation studies with a larger number of samples and including patients' clinical data are warranted. In conclusion, the QMAP system is a useful tool for simultaneously differentiating between MTBC and NTM strains as well as determining RIF- and INH-resistant MTB strains.
Acknowledgment
This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Korea (grant number: HI16C1569).
References
1. Zaman K. Tuberculosis: a global health problem. J Health Popul Nutr. 2010; 28:111–113. PMID: 20411672.
2. Bae E, Im JH, Kim SW, Yoon NS, Sung H, Kim MN, et al. Evaluation of combination of BACTEC mycobacteria growth indicator tube 960 system and Ogawa media for mycobacterial culture. Korean J Lab Med. 2008; 28:299–306. PMID: 18728380.
3. Parsons LM, Somoskövi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011; 24:314–350. PMID: 21482728.
4. Rageade F, Picot N, Blanc-Michaud A, Chatellier S, Mirande C, Fortin E, et al. Performance of solid and liquid culture media for the detection of Mycobacterium tuberculosis in clinical materials: meta-analysis of recent studies. Eur J Clin Microbiol Infect Dis. 2014; 33:867–870. PMID: 24760249.
5. Blakemore R, Story E, Helb D, Kop J, Banada P, Owens MR, et al. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol. 2010; 48:2495–2501. PMID: 20504986.
6. Skenders GK, Holtz TH, Riekstina V, Leimane V. Implementation of the INNO-LiPA Rif. TB® line-probe assay in rapid detection of multidrug-resistant tuberculosis in Latvia. Int J Tuberc Lung Dis. 2011; 15:1546–1552. PMID: 22008771.
7. Teo J, Jureen R, Chiang D, Chan D, Lin R. Comparison of two nucleic acid amplification assays, the Xpert MTB/RIF assay and the amplified Mycobacterium Tuberculosis Direct assay, for detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol. 2011; 49:3659–3662. PMID: 21865419.
8. Singhal R, Myneedu VP, Arora J, Singh N, Sah GC, Sarin R. Detection of multi-drug resistance & characterization of mutations in Mycobacterium tuberculosis isolates from North-Eastern States of India using GenoType MTBDRplus assay. Indian J Med Res. 2014; 140:501–506. PMID: 25488443.
9. Sharma SK, Kohli M, Yadav RN, Chaubey J, Bhasin D, Sreenivas V, et al. Evaluating the diagnostic accuracy of Xpert MTB/RIF assay in pulmonary tuberculosis. PLoS One. 2015; 10:e0141011. PMID: 26496123.
10. Rahman A, Sahrin M, Afrin S, Earley K, Ahmed S, Rahman SM, et al. Comparison of Xpert MTB/RIF assay and GenoType MTBDRplus DNA probes for detection of mutations associated with rifampicin resistance in Mycobacterium tuberculosis. PLoS One. 2016; 11:e0152694. PMID: 27054344.
11. Seifert M, Ajbani K, Georghiou SB, Catanzaro D, Rodrigues C, Crudu V, et al. A performance evaluation of MTBDRplus version 2 for the diagnosis of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2016; 20:631–637. PMID: 27084817.
12. Kim LN, Kim M, Jung K, Bae HJ, Jang J, Jung Y, et al. Shape-encoded silica microparticles for multiplexed bioassays. Chem Commun (Camb). 2015; 51:12130–12133. PMID: 26125980.
13. Wang HY, Uh Y, Kim S, Lee H. Performance of the Quantamatrix Multiplexed Assay Platform system for the differentiation and identification of Mycobacterium species. J Med Microbiol. 2017; 66:777–787. PMID: 28604333.
14. Wang HY, Uh Y, Kim S, Lee H. Quantamatrix Multiplexed Assay Platform system for direct detection of bacteria and antibiotic resistance determinants in positive blood culture bottles. Clin Microbiol Infect. 2017; 23:333.e1–333.e7.
15. Abbadi SH, Sameaa GA, Morlock G, Cooksey RC. Molecular identification of mutations associated with anti-tuberculosis drug resistance among strains of Mycobacterium tuberculosis. Int J Infect Dis. 2009; 13:673–678. PMID: 19138546.
16. Tessema B, Beer J, Emmrich F, Sack U, Rodloff AC. Analysis of gene mutations associated with isoniazid, rifampicin and ethambutol resistance among Mycobacterium tuberculosis isolates from Ethiopia. BMC Infect Dis. 2012; 12:37. PMID: 22325147.
17. Luo T, Zhao M, Li X, Xu P, Gui X, Pickerill S, et al. Selection of mutations to detect multidrug-resistant Mycobacterium tuberculosis strains in Shanghai, China. Antimicrob Agents Chemother. 2010; 54:1075–1081. PMID: 20008778.
18. Slayden RA, Barry CE 3rd. The genetics and biochemistry of isoniazid resistance in Mycobacterium tuberculosis. Microbes Infect. 2000; 2:659–669. PMID: 10884617.
19. Jin J, Zhang Y, Fan X, Diao N, Shao L, Wang F, et al. Evaluation of the GenoType® MTBDRplus assay and identification of a rare mutation for improving MDR-TB detection. Int J Tuberc Lung Dis. 2012; 16:521–526. PMID: 22325117.
20. Yadav RN, Singh BK, Sharma SK, Sharma R, Soneja M, Sreenivas V, et al. Comparative evaluation of GenoType MTBDRplus line probe assay with solid culture method in early diagnosis of multidrug resistant tuberculosis (MDR-TB) at a tertiary care centre in India. PLoS One. 2013; 5:e72036.
21. Causse M, Ruiz P, Gutierrez JB, Vaquero M, Casal M. New Anyplex™ II MTB/MDR/XDR kit for detection of resistance mutations in M. tuberculosis cultures. Int J Tuberc Lung Dis. 2015; 19:1542–1546. PMID: 26614199.
22. Hristea A, Otelea D, Paraschiv S, Macri A, Baicus C, Moldovan O, et al. Detection of Mycobacterium tuberculosis resistance mutations to rifampin and isoniazid by real-time PCR. Indian J Med Microbiol. 2010; 28:211–216. PMID: 20644308.
23. Chien JY, Chang TC, Chiu WY, Yu CJ, Hsueh PR. Performance assessment of the BluePoint MycoID plus kit for identification of Mycobacterium tuberculosis, including rifampin- and isoniazid-resistant isolates, and nontuberculous mycobacteria. PLoS One. 2015; 10:e0125016. PMID: 25938668.
24. Bai Y, Wang Y, Shao C, Hao Y, Jin Y. GenoType MTBDRplus Assay for rapid detection of multidrug resistance in Mycobacterium tuberculosis: a meta-analysis. PLoS One. 2016; 11:e0150321. PMID: 26934724.
Table 1
Comparison of RIF resistance between phenotypic DST and molecular detection methods for 200 clinical Mycobacterium tuberculosis isolates
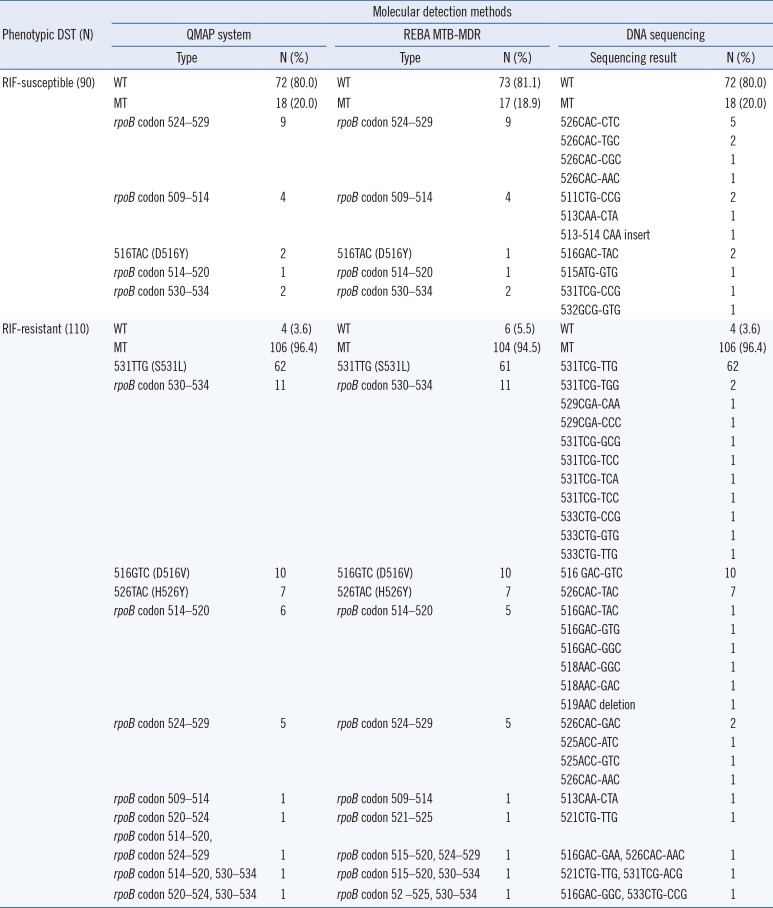
Table 2
Comparison of INH resistance between phenotypic DST and molecular detection methods using 200 clinical Mycobacterium tuberculosis isolates
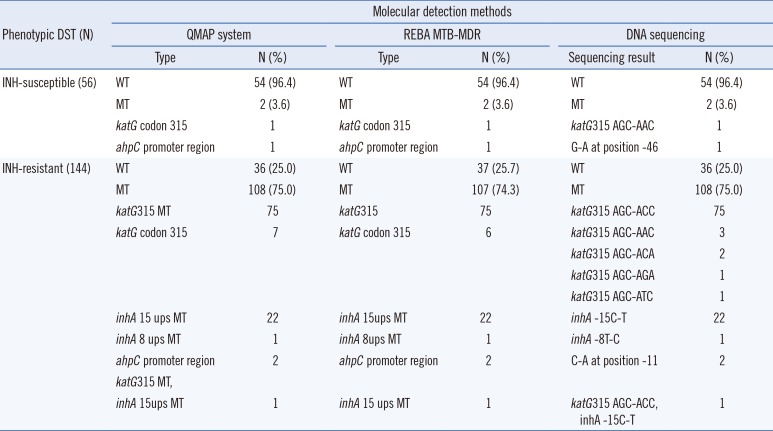
Table 3
Diagnostic performance of the QMAP system compared with phenotypic DST and DNA-sequencing
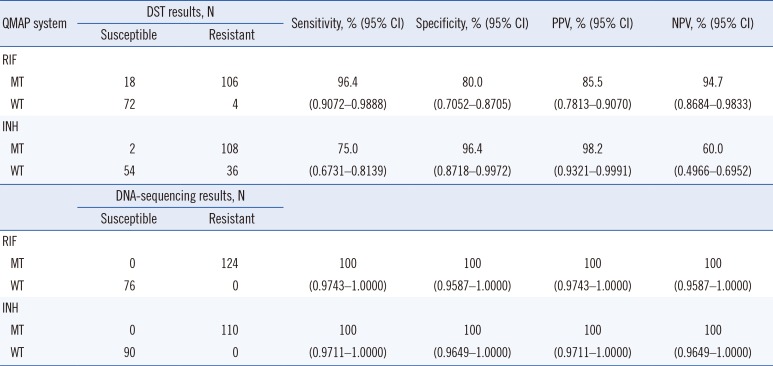
Table 4
Probes used to detect RIF- and INH-resistant MTB with QMAP and REBA MTB-MDR
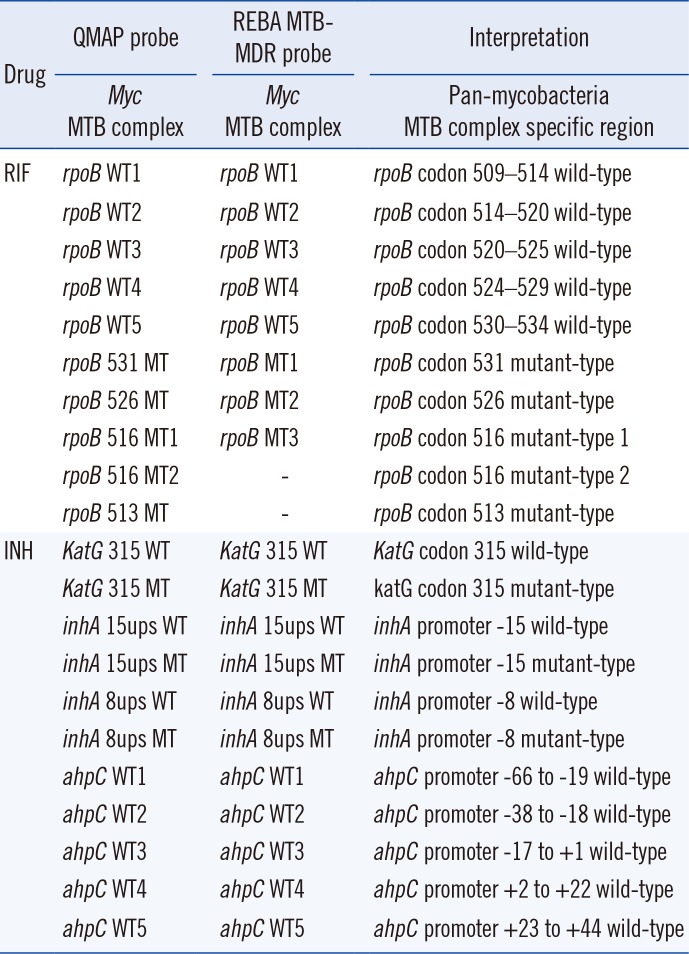
Table 5
Comparison of the QMAP system and REBA MTB-MDR assay for detection of RIF and INH resistance

| REBA MTB-MDR | QMAP system | Agreement, % (95% CI) | Kappa* | |
|---|---|---|---|---|
| MT, N | WT, N | |||
| RIF | ||||
| MT | 121 | 0 | 98.5 | 0.968 |
| WT | 3 | 76 | (0.9548–0.9969) | |
| INH | ||||
| MT | 109 | 0 | 99.5 | 0.990 |
| WT | 1 | 90 | (0.9694–1.0000) | |
Table 6
Discrepant RIF and INH resistance detection results from phenotypic DST and molecular detection methods




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download