Abstract
Background
Early detection of tuberculosis (TB) is challenging in resource-poor settings because of limited accessibility to molecular diagnostics. The aim of this study was to evaluate the performance of the loop-mediated isothermal amplification kit (TB-LAMP) for TB diagnosis compared with conventional and molecular tests.
Methods
A total of 290 consecutive sputum samples were collected from May till September, 2015. All samples were processed using the N-Acetyl-L-cysteine (NALC) NaOH method and tested by smear microscopy, solid and liquid culture, real-time PCR, and TB-LAMP.
Results
The sensitivity of TB-LAMP for smear-positive and smear-negative samples with culture positivity was 92.0% and 58.8%, respectively. TB-LAMP was positive in 14.9% of TB culture-negative samples; however, all those samples were also positive by real-time PCR. In addition, none of the samples positive for nontuberculous mycobacteria by culture were positive by TB-LAMP. The overall agreement between TB-LAMP and real-time PCR was good; however, the concordance rate was significantly lower for real-time PCR positive samples with Ct values of 30–35.
Tuberculosis (TB) is a major global health threat. According to the WHO, 9.6 million people newly developed TB, and 1.5 million died from TB in 2014 [1]. Successful control of TB necessitates its timely and correct diagnosis. However, a large proportion of TB cases are missed by TB control programs, and even notified TB patients often do not undergo any type of laboratory test, especially in resource-poor settings [1]. Thus, laboratory improvements and new diagnostic tools are crucial for identifying more TB cases and improving the accuracy of TB diagnosis. While current microbiological tests, including smear microscopy and culture, have been used as standard TB diagnosis methods, molecular tests are considered as alternative diagnostics for overcoming the limitations of conventional tests [2]. Many countries have invested considerable effort in implementing new diagnostics; however, only a few molecular methods are available in high-TB burden countries. Moreover, most molecular tests are expensive and complicated and thus are not feasible at the district level where most TB patients are cared for [3].
Loop-mediated isothermal amplification (LAMP) is a unique nucleic acid amplification test (NAAT) [4]. This method has been applied to the diagnostics of various infectious diseases [5]. Eiken Chemical developed a diagnostic kit for TB using the LAMP method (TB-LAMP) [6]. TB-LAMP has been highlighted as a candidate replacement for conventional TB diagnosis tests because of its simplicity and low cost [3]. There have been many studies evaluating LAMP for TB diagnosis. However, early studies often used in-house kits, and only few studies compared TB-LAMP with both conventional and molecular tests [7]. The aims of this study were to evaluate the performance of TB-LAMP compared with conventional and molecular tests and assess its feasibility as a replacement test for smear microscopy.
A total of 290 consecutive sputum samples were collected from 247 patients who visited public health centers from May till September, 2015. All samples were tested by smear microscopy, culture, real-time PCR, and TB-LAMP at the Korean Institute of Tuberculosis, which is a supranational TB reference laboratory. The protocol was reviewed by the public institutional review board designated by the Ministry of Health and Welfare of the Republic of Korea (P01-201511-31-003).
Samples were processed using the N-Acetyl-L-cysteine (NALC) NaOH method according to the WHO guidelines [8]. Briefly, NALC-2% NaOH solution (at a volume equal to that of the sample) was added to the sample tube and mixed by vortexing. Following 15 minutes incubation at room temperature (20–25℃), the sample was centrifuged at 3,000 g for 15 minutes. Next, the supernatant was poured off, and the pellet was resuspended with 2 mL of phosphate buffer. Smears were prepared from the pellet and stained with auramine O. Slides were examined using fluorescence microscopy, and the results were recorded according to the WHO guidelines [8]. Culture was performed using the MGIT 960 system (BD, Sparks, MD, USA) with Lowenstein-Jensen medium. If a culture was contaminated, stored samples were processed again using the same method and inoculated into a new culture medium. Differentiation of Mycobacterium tuberculosis (MTB) in positive cultures was performed using the SD Bioline TB Ag MPT64 RAPID kit (Standard Diagnostics, Seoul, Korea).
Real-time PCR was performed using the Advansure TB/NTM kit (LG Life Sciences, Seoul, Korea) according to the manufacturer's instructions. DNA was extracted using the bead-beating method, as previously described [9]. DNA template was added to the reaction tube and loaded onto a SLAN system (LG Life Sciences). This kit detects both MTB and nontuberculous mycobacteria (NTM). Cycle threshold (Ct) values were recorded, as well as MTB-positive results.
The TB-LAMP test was conducted following the manufacturer's instructions. Briefly, DNA was extracted using the Loopamp PURE DNA extraction kit (Eiken Chemical Co., Ltd., Tokyo, Japan). Following pretreatment of the sputum sample, 60 µL of pellet was transferred to the heating tube of the kit and incubated at 90℃ for 5 minutes. Then, the heating tube was assembled with the absorbent tube, and the sample was mixed with an absorbent powder by vigorous shaking. After attaching the injection cap to the tube, 30 µL of the DNA solution was dispensed into the reaction tube of the Loopamp MTBC detection kit (Eiken Chemical). The tubes were loaded into a turbidimeter (LA500, Eiken Chemical) at 67℃ for 40 minutes.
Statistical analyses were carried out using the Analyse-it program (Analyse-it software, Leeds, UK). Test agreement was presented as percent agreement and kappa coefficient. The Wilcoxon-Mann-Whitney test was used to analyze the differences in the Ct values of the real-time PCR by TB-LAMP results between real-time PCR positive samples. A P value <0.05 was considered statistically significant.
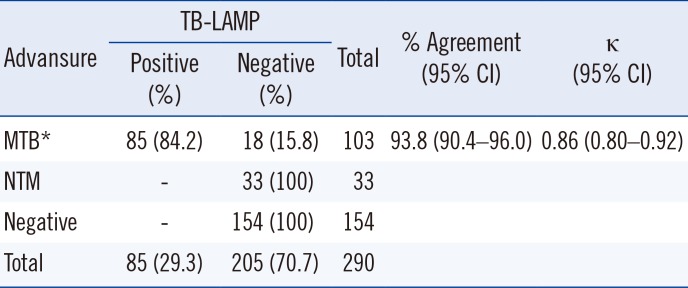
Of the 290 samples, 186 (64.1%) were smear-negative and 104 (35.9%) were smear-positive (Table 1). TB-LAMP detected MTB in 14 of the 186 smear-negative samples and in 71 of the 104 smear-positive samples (Table 1). The positive rate of TB-LAMP increased with smear microscopy grade. TB-LAMP was positive in 17.6%, 73.6%, 81.3%, and 88.9% of scanty, 1+, 2+, and 3+ samples, respectively. There were 67 culture-positive samples, of which 56 (83.6%) were positive by TB-LAMP (Table 2). Of the 186 smear-negative samples, 17 cases of MTB were identified by culture, 10 of which (58.8%) were detected by TB-LAMP (Table 2). MTB was cultured from 50 of the 104 smear-positive samples, and TB-LAMP was positive in 46 (92.0%) of these samples (Table 2). Thirty-three samples were culture-positive for NTM; none of them were positive by TB-LAMP or real-time PCR. Of the 188 culture-negative samples, 28 samples were positive by TB-LAMP and real-time PCR. After excluding these samples, the sensitivity, specificity, positive predictive value, and negative predictive value compared with culture were 83.6%, 100%, 100%, and 94.6%, respectively (Table 3). In addition, the sensitivity of TB-LAMP for smear-positive and negative samples was 92.0% and 58.8%, respectively. The concordance rate between TB-LAMP and real-time PCR was fairly high (93.8% agreement, κ=0.86; Table 4). MTB was not detected by TB-LAMP in all samples identified as MTB-negative or NTM-positive by real-time PCR. Of the 103 MTB-positive samples by real-time PCR, the positive rate for TB-LAMP was 84.2% (85/103). However, the median real-time PCR Ct values were significantly different between the TB-LAMP positive and negative groups (Fig. 1). The TB-LAMP positive rate was 98.7% (78/79) for high MTB-positive samples with Ct values <30. In contrast, TB-LAMP failed to detect MTB in 70.8% (17/24) of samples with low MTB content (Ct value 30 to 35).
The overall performance of TB-LAMP was superior to smear microscopy in this study. TB-LAMP detected MTB in 10 out of 17 smear-negative/culture-positive samples; thus, the sensitivity for those samples was 58.8% (Table 3). The sensitivity of TB-LAMP for smear-positive samples was 92.0% (Table 3). Several evaluation studies have been conducted in various countries. However, the performance indicators varied greatly across the studies [10111213]. Previous studies have shown that TB-LAMP has lower sensitivity than fluorescence microscopy (FM) and that the performance of TB-LAMP is insufficient in smear-negative samples [1415]. However, in this study, TB-LAMP detected more TB cases than FM. Although culturing was used as the standard method in this study and is more sensitive than any other method, culturing may fail because of contamination. The optimal contamination rate of solid media culture is 2% to 5% and is higher for liquid media [7]. Although decontamination was repeated if a culture was contaminated, contamination still occurred during culturing. In such cases, NAAT would be useful, and TB-LAMP actually detected MTB in samples with culture contamination. Ou et al [12] reported a recent large-scale multicenter study on TB-LAMP conducted in China. They showed that the sensitivity of TB-LAMP was 92.12% and 53.81% for smear-positive and smear-negative samples with positive culture, respectively. These results were comparable to our findings.
Many real-time PCR-based assays have been developed and are commonly used to detect MTB and drug resistance to anti-TB drugs [916171819]. Generally, the sensitivity of real-time PCR assays is higher than that of TB-LAMP [617181920]. The detection rates of TB-LAMP have been shown to be clearly distinguishable with a real-time PCR Ct value of 30. TB-LAMP was positive for 98.7% of samples with Ct<30; however, the positive rate decreased to 29.2% for samples with Ct values of 30 to 35. It indicates that TB-LAMP is inferior to real-time PCR in terms of analytic sensitivity. This may be due to a difference in amplification efficiency between the isothermal test and real-time PCR. In addition, a relatively small volume (60 µL) of the sample is utilized for TB-LAMP compared with other NAATs. TB-LAMP was found to be negative for four samples that were positive by both smear microscopy and culture. These samples were all scanty-positive by smear, and their real-time PCR Ct values were >30. Nevertheless, negative TB-LAMP results in smear-positive samples should not be ignored and require further evaluation.
Mitarai et al [20] reported that indirect LAMP using concentrated samples showed lower sensitivity than direct application, especially for smear-negative samples. Our study tested concentrated samples only, so we could not evaluate the difference in sensitivity between indirect and direct testing. However, the sensitivity of TB-LAMP for smear-negative samples was similar to that of direct LAMP testing reported by Mitarai et al [20]. Therefore, further evaluation is necessary to determine which approach is better for TB-LAMP.
In this study, a large proportion of culture-negative samples were found to be positive by TB-LAMP. We were unable to reveal the reason for this because the tests were requested without clinical information including diagnosis, X-ray findings, and previous laboratory results. This is a major limitation of our study. We speculate that the suspected causes are delayed sample delivery and patient TB medication. In fact, those discrepant samples were all confirmed to be positive by real-time PCR; thus, we concluded that they were not false positive results. Previous studies conducted in Korea have demonstrated a similar problem of high NAAT-positive rates for culture-negative samples [2122]. All discrepant cases or samples from cases with a recent TB medication history were excluded from analysis in these studies [2122]. NTM can be isolated from culture. The prevalence of NTM infection has increased in low-TB burden countries, and NTM is frequently isolated from TB suspects even in high-TB burden countries [2324]. NAATs are useful for differentiating between MTB and NTM in smear-positive samples [16]. In this study, NTM was isolated from 33 samples, none of which were positive by TB-LAMP.
NAAT has many advantages over conventional microbiological tests such as smear microscopy and culture [3]. The turnaround time (TAT) of NAATs is much faster than that of culture, and the sensitivity and specificity of NAATs are significantly higher than those of smear microscopy. However, NAATs have not been widely used in high-TB burden, resource-poor settings, because they require infrastructure and trained staff [25]. To date, only two NAATs (Xpert MTB/RIF and TB-LAMP) were endorsed by the WHO for the diagnosis of pulmonary TB [2627]. Xpert has been implemented in many countries following the WHO policy [226]. However, many factors need to be considered prior to implementing Xpert. Rifampicin susceptibility testing is usually not necessary for new cases, and Xpert may cause false resistance results in settings with low rifampicin resistance rates [26]. Moreover, the GeneXpert instrument is vulnerable to unstable electricity supply and harsh environments; Xpert test failures and module malfunction are more common in settings with poor infrastructure [28]. TB-LAMP might be less sensitive than Xpert, but it is less affected by electricity and temperature [27]. While most NAATs require a separate DNA extraction step, the TB-LAMP system provides the solution for DNA extraction (Loopamp PURE DNA extraction kit) [20]. In addition, amplification can be detected by visual inspection under UV light instead of using a turbidimeter (LA-500). Compared with existing NAATs, TB-LAMP would be more feasible for district level laboratories, which are the basic unit of laboratory network and case management [529].
Thus, TB-LAMP could be more feasible at the district level in resource-limited countries. In addition, TB laboratories can process more samples using TB-LAMP than smear microscopy or Xpert because of its fast TAT and higher throughput [2627]. In conclusion, this study demonstrates that TB-LAMP has decent performance compared with conventional and molecular tests. Therefore, it could replace smear microscopy in the future and be useful in increasing TB diagnostic capacity in situations where Xpert is not feasible because of poor infrastructure.
Acknowledgements
This study was supported by Shinyang Chemical (Seoul, Korea). The sponsor had no involvement in the study setting, data interpretation, or writing of the manuscript. The authors wish to thank Dr. Yong Jung Park for his assistance with the statistical analysis.
References
1. World Health Organization. Global tuberculosis report. 2015.
2. Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nat Rev Dis Primers. 2016; 2:16076. PMID: 27784885.
3. Parsons LM, Somoskovi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011; 24:314–350. PMID: 21482728.
4. Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000; 28:E63. PMID: 10871386.
5. Notomi T, Mori Y, Tomita N, Kanda H. Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol. 2015; 53:1–5. PMID: 25557475.
6. Bojang AL, Mendy FS, Tientcheu LD, Otu J, Antonio M, Kampmann B, et al. Comparison of TB-LAMP, GeneXpert MTB/RIF and culture for diagnosis of pulmonary tuberculosis in The Gambia. J Infect. 2016; 72:332–337. PMID: 26724771.
7. Nagai K, Horita N, Yamamoto M, Tsukahara T, Nagakura H, Tashiro K, et al. Diagnostic test accuracy of loop-mediated isothermal amplification assay for Mycobacterium tuberculosis: systematic review and meta-analysis. Sci Rep. 2016; 6:39090. PMID: 27958360.
8. Global Laboratory Initiative. Mycobacteriology Laboratory Manual. Geneva, Switzerland: Global Laboratory Initiative;2014.
9. Ko Y, Lee HK, Lee YS, Kim MY, Shin JH, Shim EJ, et al. Accuracy of Xpert® MTB/RIF assay compared with AdvanSure™ TB/NTM real-time PCR using bronchoscopy specimens. Int J Tuberc Lung Dis. 2016; 20:115–120. PMID: 26688537.
10. Boehme CC, Nabeta P, Henostroza G, Raqib R, Rahim Z, Gerhardt M, et al. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. J Clin Microbiol. 2007; 45:1936–1940. PMID: 17392443.
11. Kaku T, Minamoto F, D'Meza R, Morose W, Boncy J, Bijou J, et al. Assessment of Accuracy of LAMP-TB Method for Diagnosing Tuberculosis in Haiti. Jpn J Infect Dis. 2016; 69:488–492. PMID: 27000457.
12. Ou X, Li Q, Xia H, Pang Y, Wang S, Zhao B, et al. Diagnostic accuracy of the PURE-LAMP test for pulmonary tuberculosis at the county-level laboratory in China. PLoS One. 2014; 9:e94544. PMID: 24788724.
13. Yoshida H, Onohara K, Tazawa T, Tsurinaga Y, Kurokawa M, Han Y, et al. Comparative study of the efficacy of the COBAS TaqMan and LAMP assay for the rapid diagnosis of tuberculosis. Kekkaku. 2013; 88:727–733. PMID: 24432481.
14. George G, Mony P, Kenneth J. Comparison of the efficacies of loop-mediated isothermal amplification, fluorescence smear microscopy and culture for the diagnosis of tuberculosis. PLoS One. 2011; 6:e21007. PMID: 21695047.
15. Nliwasa M, MacPherson P, Chisala P, Kamdolozi M, Khundi M, Kaswaswa K, et al. The sensitivity and specificity of loop-mediated isothermal amplification (LAMP) assay for tuberculosis diagnosis in adults with chronic cough in Malawi. PLoS One. 2016; 11:e0155101. PMID: 27171380.
16. Jung YJ, Kim JY, Song DJ, Koh WJ, Huh HJ, Ki CS, et al. Evaluation of three real-time PCR assays for differential identification of Mycobacterium tuberculosis complex and nontuberculous mycobacteria species in liquid culture media. Diagn Microbiol Infect Dis. 2016; 85:186–191. PMID: 27105774.
17. Yang B, Wang X, Li H, Li G, Cao Z, Cheng X. Comparison of loop-mediated isothermal amplification and real-time PCR for the diagnosis of tuberculous pleurisy. Lett Appl Microbiol. 2011; 53:525–531. PMID: 21883320.
18. Kim MJ, Nam YS, Cho SY, Park TS, Lee HJ. Comparison of the Xpert MTB/RIF assay and real-time PCR for the detection of Mycobacterium tuberculosis. Ann Clin Lab Sci. 2015; 45:327–332. PMID: 26116598.
19. Cho WH, Won EJ, Choi HJ, Kee SJ, Shin JH, Ryang DW, et al. Comparison of AdvanSure TB/NTM PCR and COBAS TaqMan MTB PCR for detection of Mycobacterium tuberculosis complex in routine clinical practice. Ann Lab Med. 2015; 35:356–361. PMID: 25932446.
20. Mitarai S, Okumura M, Toyota E, Yoshiyama T, Aono A, Sejimo A, et al. Evaluation of a simple loop-mediated isothermal amplification test kit for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2011; 15:1211–1217. PMID: 21943848.
21. Kwak N, Choi SM, Lee J, Park YS, Lee CH, Lee SM, et al. Diagnostic accuracy and turnaround time of the Xpert MTB/RIF assay in routine clinical practice. PLoS One. 2013; 8:e77456. PMID: 24204834.
22. Huh HJ, Jeong BH, Jeon K, Koh WJ, Ki CS, Lee NY. Performance evaluation of the Xpert MTB/RIF assay according to its clinical application. BMC Infect Dis. 2014; 14:589. PMID: 25395048.
23. Ley S, Carter R, Millan K, Phuanukoonnon S, Pandey S, Coulter C, et al. Non-tuberculous mycobacteria: baseline data from three sites in Papua New Guinea, 2010-2012. Western Pac Surveill Response J. 2015; 6:24–29. PMID: 26798558.
24. Aliyu G, El-Kamary SS, Abimiku A, Brown C, Tracy K, Hungerford L, et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One. 2013; 8:e63170. PMID: 23671669.
25. Gray CM, Katamba A, Narang P, Giraldo J, Zamudio C, Joloba M, et al. Feasibility and operational performance of tuberculosis detection by loop-mediated isothermal amplification platform in decentralized settings: Results from a multicenter study. J Clin Microbiol. 2016; 54:1984–1991. PMID: 27194691.
26. World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children: Policy update. Geneva: World Health Organization;2013.
27. World Health Organization. The use of loop-mediated isothermal amplification (TB-LAMP) for the diagnosis of pulmonary tuberculosis: Policy guidance. Geneva: World Health Organization;2016.
28. Creswell J, Codlin AJ, Andre E, Micek MA, Bedru A, Carter EJ, et al. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect Dis. 2014; 14:2. PMID: 24383553.
29. Geojith G, Dhanasekaran S, Chandran SP, Kenneth J. Efficacy of loop mediated isothermal amplification (LAMP) assay for the laboratory identification of Mycobacterium tuberculosis isolates in a resource limited setting. J Microbiol Methods. 2011; 84:71–73. PMID: 21047534.
Fig. 1
Distribution of Ct values of real-time PCR according to TB-LAMP results. Median Ct values for TB-LAMP-positive (n=79) and negative (n=24) samples were 24.5 and 31.1, respectively (P<0.0001).
Abbreviations: TB-LAMP, loop-mediated isothermal amplification assay.
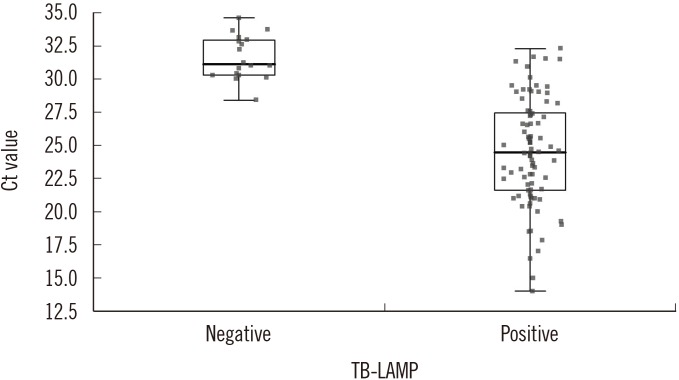
Table 1
Comparison of TB-LAMP positive rates according to smear grade
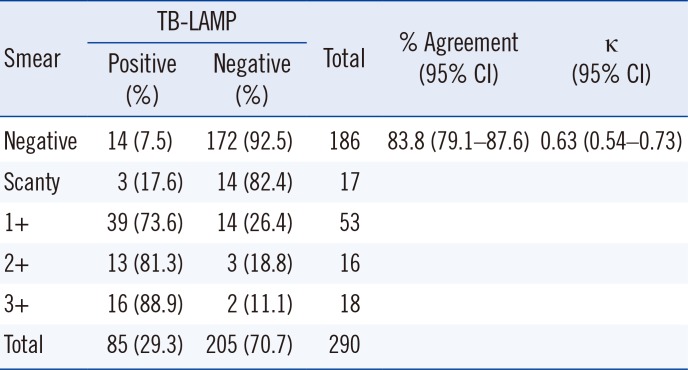
Table 2
Comparison of the TB-LAMP and culture according to AFB smear
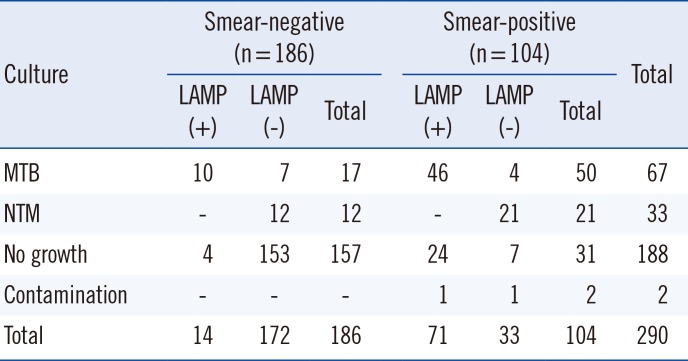
Table 3
Diagnostic accuracy of the TB-LAMP compared with culture
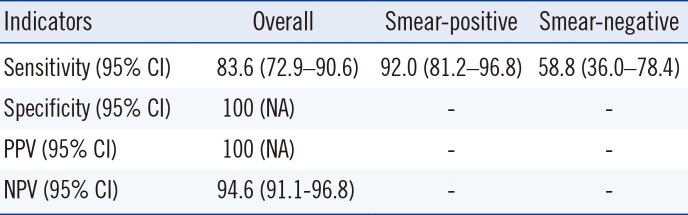
Twenty-eight culture-negative samples that were positive by both TB-LAMP and real-time PCR were excluded from the analysis.
Abbreviations: TB-LAMP, loop-mediated isothermal amplification assay; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; NA, not available.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download