Dear Editor,
Pasteurella canis is a small, gram-negative coccobacillus that is a part of the normal flora of healthy domestic animals, especially dogs [1]. Although P. canis is a well-known major pathogen of infections caused by dog bites, only few cases of human infections with P. canis have been reported [2345]. We report a case of soft tissue infection caused by P. canis.
A 54-yr-old woman without underlying diseases visited the emergency department with multiple lacerations caused by a dog bite incurred two days prior. She had received immediate treatment with wound dressing, antibiotics (amoxicillin/clavulanic acid), and tetanus immunoglobulin at another hospital on the day she was bitten. However, the wound was worsening. The initial vital signs were as follows: temperature, 37.7℃; pulse rate, 88/min; respiratory rate, 20/min; blood pressure 108/73 mmHg. The wounds were located on the right distal arm (3 cm in the anteromedial area, 3 cm in the anterolateral area, and 4 cm in the posterior area with muscle exposure). Abnormalities such as fracture were absent on plain radiographs.
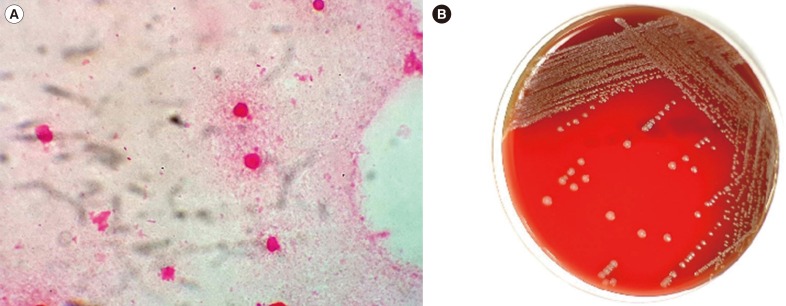
The initial blood counts were as follows: white blood cells (WBCs), 10.4×109/L (neutrophils, 80.5%); hemoglobin, 12.4 g/dL; and platelets 173×109/L. No prominent abnormalities in blood chemistry were observed on routine tests except for mildly elevated acute phase reactants (C-reactive protein [CRP], 5.82 mg/dL; erythrocyte sedimentation rate [ESR], 52 mm/hr). The patient was admitted, and amoxicillin/clavulanic acid were administered immediately and maintained throughout the hospitalization period. Tetanus and rabies vaccines were also given to prevent potential infections. Microbiological examination of the wounds was performed. A specimen was collected for Gram staining and culture. Gram-negative coccobacilli were observed on a direct gram-stained smear from the wound specimens (Fig. 1A). Pus specimens were inoculated on 5% sheep blood agar, chocolate agar, and MacConkey agar. After 24 hr, the growth of smooth, grayish-white colonies was observed on the blood agar and chocolate agar but not on the MacConkey agar (Fig. 1B). The colonies were positive for catalase and oxidase reactions. All isolates were identified with MicroScan Walkaway (Beckman Coulter, Brea, CA, USA) and Vitek automated bacterial identification systems 2 (bioMérieux, Marcy-l'Etoile, France) and a VITEK MS matrix-assisted laser desorption ionization mass spectrometry (MALDI-TOF MS) system (bioMérieux).
For further identification of bacterial species, 16S ribosomal RNA (rRNA) gene sequencing was carried out. The universal primers 8F (5´-TTGGAGAGTTTGATCCTGGCTC-3´) and 801R (5´-GGCGTGGACTTCCAGGGTATCT-3´) were used to amplify parts of the 16S rRNA gene. The VITEK MS and Vitek 2 (bioMérieux) analyses identified P. canis, whereas the MicroScan Walkaway analysis identified Pasteurella multocida. The 16S rRNA gene sequences of the strain in this study were most closely related to those of the P. canis type strain. The identity was 99.1% with strain CCUG 12400 (GenBank accession number AY362919).
An antimicrobial susceptibility test was performed by using the CLSI disk diffusion method [6]. The isolate was susceptible to ampicillin (zone diameter, 27 mm), penicillin (30 mm), tetracycline (30 mm), ceftriaxone (34 mm), ciprofloxacin (29 mm), and trimethoprim/sulfamethoxazole (24 mm). On hospital day 3, secondary wound closure with surgical abscess drainage and debridement was performed, and the patient was discharged on hospital day 12. One week later, the treatment was completed with removal of the stitches at the outpatient clinic.
Approximately 15-20% of dog bite wounds and ≥50% of cat bite wounds contain a mixture of aerobic and anaerobic bacteria reflecting the oral flora of the biting animal [7]. Studying dog bites, Talan et al [1] reported that the most commonly identified aerobic and anaerobic bacteria are Pasteurella spp. (50%), Streptococcus spp. (46%), Staphylococcus spp. (46%), Fusobacterium spp. (32%), and Bacteroides spp. (30%). The same study identified P. canis in 26% of dog bites, which is a relatively high rate. However, this species has typically been overlooked in the clinical setting because gram-negative bacilli do not grow on MacConkey agar. In the present study, MicroScan Walkaway (Beckman Coulter) analysis identified the bacteria as P. multocida owing to the absence of a P. canis database.
Empirical antibiotics for dog and cat bites should cover Pasteurella spp., Streptococcus spp., Staphylococcus spp., and anaerobes. Therefore, β-lactam/β-lactamase inhibitors, a second-generation cephalosporin with anaerobic activity, or combination therapy with either penicillin and a first-generation cephalosporin or clindamycin and a fluoroquinolone are recommended [1]. Pasteurella spp. are generally susceptible to most antibiotics, including penicillin G, amoxicillin/clavulanic acid, piperacillin/tazobactam, doxycycline, fluoroquinolones, advanced cephalosporins, and carbapenems [8910]. Given this information, we chose amoxicillin/clavulanic acid for the initial treatment of this patient.
This report is the first of P. canis isolation from a dog bite wound in a human in Korea. The results of this study emphasize the importance of proper laboratory identification for the diagnosis and treatment of infections caused by animal bites.
Acknowledgments
This study was supported by the research fund of Hanyang University (HY-201400000002367).
References
1. Talan DA, Citron DM, Abrahamian FM, Moran GJ, Goldstein EJ. Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N Engl J Med. 1999; 340:85–92. PMID: 9887159.
2. Albert TJ. The first case of Pasteurella canis bacteremia: a cirrhotic patient with an open leg wound. Infection. 2010; 38:483–485. PMID: 20623245.
3. Castellano I, Marín JP, Gallego S, Mora M, Rangel G, Suarez MA, et al. Pasteurella canis peritonitis in a peritoneal dialysis patient. Perit Dial Int. 2011; 31:503–504. PMID: 21799062.
4. Hara H, Ochiai T, Morishima T, Arashima Y, Kumasaka K, Kawano KY. Pasteurella canis osteomyelitis and cutaneous abscess after a domestic dog bite. J Am Acad Dermatol. 2002; 46:S151–S152. PMID: 12004298.
5. Hazelton BJ, Axt MW, Jones CA. Pasteurella canis osteoarticular infections in childhood: review of bone and joint infections due to pasteurella species over 10 years at a tertiary pediatric hospital and in the literature. J Pediatr Orthop. 2013; 33:e34–e38. PMID: 23482278.
6. Clinical and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. Approved guideline, M45-A2. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2010.
7. Abrahamian FM, Goldstein EJ. Microbiology of animal bite wound infections. Clin Microbiol Rev. 2011; 24:231–246. PMID: 21482724.
8. Lion C, Conroy MC, Carpentier AM, Lozniewski A. Antimicrobial susceptibilities of Pasteurella strains isolated from humans. Int J Antimicrob Agents. 2006; 27:290–293. PMID: 16564680.
9. Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014; 59:147–159. PMID: 24947530.
10. Citron DM, Warren YA, Fernandez HT, Goldstein MA, Tyrrell KL, Goldstein EJ. Broth microdilution and disk diffusion tests for susceptibility testing of Pasteurella species isolated from human clinical specimens. J Clin Microbiol. 2005; 43:2485–2488. PMID: 15872290.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download