Abstract
Background
Resistance of Mycobacterium tuberculosis to anti-tuberculosis (TB) drugs is almost exclusively due to spontaneous chromosomal mutations in target genes. Rapid detection of drug resistance to both first- and second-line anti-TB drugs has become a key component of TB control programs. Technologies that allow rapid, cost-effective, and high-throughput detection of specific nucleic acid sequences are needed. This study was to develop a high-throughput assay based on allele-specific primer extension (ASPE) and MagPlex-TAG microspheres to detect anti-TB drug resistance mutations.
Methods
DNA samples from 357 M. tuberculosis clinical isolates and H37Rv were amplified by multiplex PCR using four primer sets, followed by multiplex ASPE using 23 TAG-ASPE primers. The products were sorted on the TAG-ASPE array and detected by using the Luminex xMAP system. Genotypes were also determined by sequencing.
Results
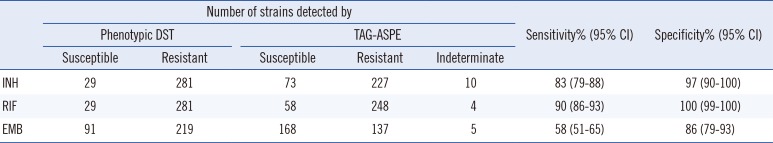
Genetic drug susceptibility typing by the TAG-ASPE method was 100% concordant with those obtained by sequencing. Compared with phenotypic drug susceptibility testing (DST) as a reference method, the sensitivity and specificity of the TAG-ASPE method were 83% (95% confidence interval [CI], 79-88%) and 97% (95% CI, 90-100%) for isoniazid. For rifampin testing, the sensitivity and specificity were 90% (95% CI, 86-93%) and 100% (95% CI, 99-100%). Also, the sensitivity and specificity were 58% (95% CI, 51-65%) and 86% (95% CI, 79-93%) for ethambutol.
Tuberculosis (TB) remains an international health problem. Many efforts have been made to control the disease since it was declared a global emergency 20 yr ago. In 2012, there were estimated 8.6 million new TB cases and 1.3 million TB deaths globally [1]. The increasing incidence of TB due to multidrug-resistant strains of Mycobacterium tuberculosis (MDR-TB, defined as resistance to at least rifampin [RIF] and isoniazid [INH]) is hampering efforts for the control and management of TB [23]. Among these deaths there were an estimated 170,000 from MDR-TB, a relatively high total compared with 450,000 incident cases of MDR-TB [1].
Globally, an estimated 3.6% of new cases and 20.2% of previously treated cases are due to MDR-TB. The highest levels are in eastern Europe and central Asia, where more than 20% of new cases and more than 50% of previously treated cases involve MDR-TB [1].
Molecular mechanisms of M. tuberculosis drug resistance are well known and reported to involve mutations in specific genes. RIF resistance involves mutations in the rpoB gene (the 81-bp RIF resistance-determining region [RRDR]), and INH resistance is associated with mutation in inhA (positions -15 and -8 in the inhA promoter sequence), katG (codon 315) and other genes [45678910]. Resistance to ethambutol (EMB), a first-line drug that is used in combination with INH and RIF, involves mutations in embB (codon 306) [1112].
Mutations in the 81-bp RRDR of the rpoB gene are found in about 96% of RIF-resistant M. tuberculosis isolates. Mutations at codon 516, 526, and 531 are among the most frequent mutations in RIF-resistant strains. For INH-resistant clinical isolates, mutations within the katG gene occur most frequently between codons 138 and 328, particularly at codon 315, accounting for 50-95%. Mutations at positions -15 and -8 in the inhA regulatory region also vary greatly for 6-43%. The embB codon 306 mutation is most frequent in clinical isolates resistant to EMB, accounting for as high as 65% resistant strains [13].
Rapid detection of drug resistance to both first- and second-line anti-TB drugs has become a key component of TB control programs. Technologies that allow rapid, cost-effective, and high-throughput detection of specific nucleic acid sequences are required.
There are currently three commercially available PCR-based tests for the rapid detection of drug resistance in TB: the INNO-LiPA Rif-TB (Innogenetics, Zwijndrecht, Belgium), Genotype MTBDRplus (Hain Lifescience GmbH, Nehren, Germany), and Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) assays [141516].
The Luminex xMAP system (Luminex Corp, Austin, TX, USA) is a multiplexed microsphere-based suspension array platform capable of analyzing and reporting up to 100 different reactions in a single reaction vessel and offers a new platform for high-throughput nucleic acid detection, which is being used in a variety of applications [1718]. This system can rapidly detect multiple DNA sequences and uses microsphere sets coupled to a tag that recognizes and binds the amplicon carrying the specific single nucleotide polymorphism (SNP). Each microsphere set is characterized by a distinct spectral address given by the combination of red and infrared fluorophores within the microspheres. Once bound, the target DNA molecules are fluorescently tagged with streptavidin-R-phycoerythrin, and the microspheres are individually analyzed with a red laser that recognizes the microsphere set and a green laser that provides a quantitative readout of the bound target. Some benefits of suspension array technology include rapid data acquisition, excellent sensitivity and specificity, and multiplexed analysis capability [1920].
This study was undertaken to develop a high-throughput assay based on allele-specific primer extension (ASPE) and MagPlex-TAG microspheres to detect anti-TB drug resistance mutations.
The 357 M. tuberculosis clinical isolates were obtained from the culture collection at the Korean Institute of Tuberculosis. The laboratory reference strain M. tuberculosis H37Rv, which is susceptible to all drugs and which has no mutation in any of the drug-resistant genes, was used as the wild-type control. The 309 of M. tuberculosis clinical isolates and H37Rv had the known results for phenotypic drug susceptibility by the absolute concentration method with Lowenstein-Jensen (LJ) medium. Their critical concentrations for resistance were as follows: INH, 0.2 µg/mL; RIF, 40 µg/mL; EMB, 2 µg/mL [21]. Other 48 clinical strains were included in order to further confirm the ability to detect mutations. The sequencing analysis of drug-resistant genes (inhA promoter, katG, rpoB, and embB) indicated that the mutation patterns represented the drug-resistant mutation types. A loop of fresh M. tuberculosis culture on LJ media was suspended in 400 µL of TE buffer (10 mM Tris-HCl, pH 8.0; 1 mM EDTA) in a screw cap microcentrifuge tube. After boiling the mixture at 100℃ for 15 min, heat-killed bacteria were centrifuged at 16,000g for 1 min, and the extracted DNA was stored at -20℃ for use.
Multiplex PCR reaction consisted of 50 ng genomic DNA, 1× PCR reaction buffer, 1.5 mM MgCl2, 200 µM each dNTP, 0.2 µM each primer, and 2.5 Units HotStar Taq polymerase (Qiagen, Hilden, Germany). A non-target PCR negative control was included in each assay run. PCR primer sequences are given in Table 1. Amplification condition were as follows according to the protocol by the manufacturer: 95℃ for 15 min; 40 cycles of 94℃ for 30 sec, 55℃ for 30 sec, and 72℃ for 30 sec; and a final extension at 72℃ for 7 min. Samples were kept at 4℃ until use. Multiplex PCR products were analyzed by gel electrophoresis. Four clearly resolved bands ranged in size from 170 to 250 bp under optimum PCR conditions (data not shown).
Before the ASPE reaction, each PCR reaction was treated with Exonuclease I-Shrimp Alkaline Phosphatase to remove unused primers and nucleotides. To 7.5 µL of the PCR reaction mixture, 3 µL of ExoSAP-IT reagent (Affymetrix, Cleveland, OH, USA) was added. Samples were then incubated at 37℃ for 30 min, followed by 15 min incubation at 80℃ to inactivate the enzyme and kept at 4℃ until use.
Multiplex ASPE reaction was carried out by using 5 µL treated PCR product in a final volume of 20 µL. Each reaction consisted of 1× ASPE buffer (20 mM Tris-HCl, pH 8.4; 50 mM KCl), 1.25 mM MgCl2, 0.75 U Tsp DNA polymerase (Invitrogen, Carlsbad, CA, USA), 5 µM dATP, dTTP, and dGTP (Invitrogen), 5 µM biotin-dCTP (Invitrogen), and 25 nM each TAG-ASPE primer. The TAG-ASPE primers were made to target mutations capable of detection by the currently produced kits. The TAG-ASPE primer sequences are listed in Table 2, and TAG sequences were provided by Luminex Corp. The ASPE reactions were incubated at 96℃ for 2 min; 40 cycles of 94℃ for 30 sec, 55℃ for 1 min, and 74℃ for 2 min; and kept at 4℃ until use.
Each hybridization reaction was carried out with 2,500 of each set of the appropriate MagPlex-TAG microspheres (Luminex Corp.) per reaction. The microsphere mixture was diluted in 2× Tm hybridization buffer (0.4 M NaCl, 0.2 M Tris, 0.16% Triton X-100, pH 8.0) to 100 of each microsphere set per microliter and mixed by vortexing and sonication for approximately 20 sec. Twenty five microliters of the MagPlex-TAG microsphere mixture and 20 µL of distilled water were added to each well of a 96-well plate. Five microliters of each ASPE reaction was added directly to each well. The samples were denatured at 96℃ for 90 sec and incubated at 37℃ for 30 min. Samples were pelleted by placing the plate on a magnetic separator and washed twice with 1× Tm hybridization buffer (0.2M NaCl, 0.1 M Tris, 0.08% Triton X-100, pH 8.0). The microspheres were then resuspended in 75 µL of 1× Tm hybridization buffer containing 5 µg/mL streptavidin-R-phycoerythrin (Invitrogen) and incubated for 15 min at 37℃. The reactions were analyzed in 50 µL at 37℃ on Luminex 200 system.
To optimize the microsphere-based TAG-ASPE genotyping method for the detection of first-line anti-TB drug resistance mutations, we performed the TAG-ASPE method and DNA dideoxy sequencing with M. tuberculosis H37Rv DNA and obtained the same results by both methods.
Genotyping results obtained by the microsphere-based TAG-ASPE method for all samples were compared with genotyping results obtained by dideoxy dye-terminator sequencing. DNA samples not sequenced for each mutation were excluded from analysis.
The signals (median fluorescence intensity, MFI) generated for each microsphere population were used to assess the sample genotype. The genotype was determined based on the wild or mutant allelic ratio: or
Once threshold values were set, the allelic ratio was used to discriminate wild type, mutant, and indeterminate.
We calculated sensitivity, specificity, and confidence intervals (CI) of TAG-ASPE method compared with phenotypic drug susceptibility test (DST) by the absolute concentration method with LJ medium.
DNA samples from the 357 clinical isolates and H37Rv were genotyped by both methods (Table 3, Fig. 1). For the inhA gene, 268 wild-type (C) and 90 mutant (T) were observed in the -15 position by both methods. In the -16 position, 358 wild type (A) were observed by both methods. In the -8 position, 344 wild-type (T) and 10 mutant (C) were observed by both methods, and sequencing revealed four other mutants. For the codon 315 in the katG gene, 172 wild-type (AGC) and 169 mutant (ACC) were observed by both methods, and sequencing revealed 11 other mutants.
Both methods were performed to confirm mutations in the rpoB gene (the 81-bp RIF resistance-determining region [RRDR]). In codon 516, 286 wild type (GAC) and 67 mutant (GTC and TAC) were observed by both methods, and sequencing revealed three other mutants. In codon 526, 296 wild-type (CAC) and 44 mutant (TAC and GAC) were observed by both methods, and sequencing revealed 16 other mutants. In the codon 531, 183 wild type (TCG) and 171 mutant (TTG) were observed by both methods, and sequencing revealed two other mutants.
One hundred ninety six wild type (ATG) and 157 mutant (GTG, CTG, ATA, ATC, and ATT) were observed in the codon 306 for the embB gene by using both methods. Sequencing also detected one other mutant.
Each wild type or mutant represented ≥0.7 and indeterminate represented 0.3-0.7 allele ratio ranges.
When we compared the TAG-ASPE method with phenotypic DST, the sensitivity and specificity were 83% (95% CI, 79-88%) and 97% (95% CI, 90-100%) for INH. For RIF testing, the sensitivity and specificity were 90% (95% CI, 86-93%) and 100% (95% CI, 99-100%). Also, the sensitivity and specificity were 58% (95% CI, 51-65%) and 86% (95% CI, 79-93%) for EMB (Table 4).
The Luminex xMAP system is a multiplexed microsphere-based suspension array platform capable of analyzing and reporting up to 100 different reactions in a single reaction vessel. In recent years, xMAP technology has been used increasingly for multiplexed high-throughput nucleic acid detection in research, commercial, and diagnostic molecular laboratory settings. Four genotyping assay methods have used the Luminex xMAP flow cytometer platform: direct hybridization (DH), single-base extension (SBE), ASPE, and oligonucleotide ligation (OL) [22]. Lee et al. [23] compared four flow cytometric SNP detection assays; SBE and ASPE more clearly differentiated each SNP compared with DH and OL. Also, ASPE is more cost-effective and simpler than SBE on the basis of cost and labor. Their study implicated ASPE is a good method for genetic typing, which requires many markers and a high level of multiplexing.
The resistance of M. tuberculosis to anti-TB drugs is almost exclusively due to spontaneous mutations in target genes. We chose the ASPE genotyping assay for the detection of first-line anti-TB drug resistance-related genes. For the TAG-ASPE method, mutations used in the commercially available PCR-based diagnostic tests for the inhA (position -8, -15, and -16), katG (codon 315), rpoB (codon 516, 526, and 531) and embB (codon 306) genes were targeted and confirmed by sequencing. Some studies reported the detection of mutations in anti-TB drug resistance-related genes with multiplex ligation-dependent probe amplification (MLPA) method and DH in the Luminex xMAP system. Bergval et al. [24] reported a lack of specificity of the embB306 MLPA probe of a total of 13 MLPA probes for one allele associated with resistance; the embB306 MLPA probe was unable to detect this ATG to ATA codon change. The 16 direct hybridization probes used to detect mutations in anti-TB drug resistance-related genes displayed 100% concordance with sequencing [25].
In this study, INH, RIF, and EMB drug resistance mutations were detected with high sensitivity and specificity for all targeted genes by using the TAG-ASPE method. Also, the results for all 23 TAG-ASPE primers were 100% concordant with those obtained by sequencing. For the non-detected loci, the TAG-ASPE method has an advantage that allows for the entry of new SNPs and/or the removal of unnecessary SNPs in existing assays.
Compared with manual bidirectional dideoxy sequencing or next generation sequencing, the TAG-ASPE method offers shorter analysis time and potentially lower cost [26]. Sequencing the same number of samples is more tedious because multiple PCR reactions are required per genomic sample being analyzed. Analysis of the sequencing data also requires additional time. Even with automated sequencing, manual set-up of the TAG-ASPE method would compete favorably.
The final goal of this study is to develop a high-throughput assay to detect first- and second-line anti-TB drug resistance mutations. The test in this study was successfully developed for first-line anti-TB drugs. The test using the TAG-ASPE method for second-line drugs is in progress.
In conclusion, the microspheres-based TAG-ASPE method permits multiplexed querying of different nucleotides, thereby allowing multiplexing of all alleles of a particular SNP. It is a valuable tool in the highly reproducible, cost-effective, and high-throughput clinical genotyping applications for multiple alleles associated with drug resistance.
Acknowledgments
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C0891).
References
1. World Health Organization. Global tuberculosis report 2013. Geneva: World Health Organization Press;2013. Available from http://www.who.int/tb/publications/global_report/en/.
2. World Health Organization. Anti-tuberculosis drug resistance in the world. Report no. 4. WHO/HTM/TB/2008.394. Geneva: World Health Organization Press;2008. Available from http://www.who.int/tb/publications/2008/drs_report4_26feb08.pdf.
3. Zignol M, Hosseini MS, Wright A, Weezenbeek CL, Nunn P, Watt CJ, et al. Global incidence of multidrug-resistant tuberculosis. J Infect Dis. 2006; 194:479–485. PMID: 16845631.

4. Kapur V, Li LL, Hamrick MR, Plikaytis BB, Shinnick TM, Telenti A, et al. Rapid Mycobacterium species assignment and unambiguous identification of mutations associated with antimicrobial resistance in Mycobacterium tuberculosis by automated DNA sequencing. Arch Pathol Lab Med. 1995; 119:131–138. PMID: 7848059.
5. Kapur V, Li LL, Iordanescu S, Hamrick MR, Wanger A, Kreiswirth BN, et al. Characterization by automated DNA sequencing of mutations in the gene (rpoB) encoding the RNA polymerase beta subunit in rifampin-resistant Mycobacterium tuberculosis strains from New York City and Texas. J Clin Microbiol. 1994; 32:1095–1098. PMID: 8027320.
6. Moghazeh SL, Pan X, Arain T, Stover CK, Musser JM, Kreiswirth BN. Comparative antimycobacterial activities of rifampin, rifapentine, and KRN-1648 against a collection of rifampin-resistant Mycobacterium tuberculosis isolates with known rpoB mutations. Antimicrob Agents Chemother. 1996; 40:2655–2657. PMID: 8913484.
7. Musser JM. Antimicrobial agent resistance in mycobacteria: molecular genetic insight. Clin Microbiol Rev. 1995; 8:496–514. PMID: 8665467.
8. Musser JM, Kapur V, Williams DL, Kreiswirth BN, van Soolingen D, van Embden JD. Characterization of the catalase-peroxidase gene (katG) and inhA locus in isoniazid-resistant and -susceptible strains of Mycobacterium tuberculosis by automated DNA sequencing: restricted array of mutations associated with drug resistance. J Infect Dis. 1996; 173:196–202. PMID: 8537659.
9. Telenti A, Imboden P, Marchesi F, Schmidheini T, Bodmer T. Direct, automated detection of rifampin-resis tant Mycobacterium tuberculosis by polymerase chain reaction and single-strand conformation polymorphism analysis. Antimicrob Agents Chemother. 1993; 37:2054–2058. PMID: 8257122.
10. Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992; 358:591–593. PMID: 1501713.
11. Ramaswamy SV, Amin AG, Göksel S, Stager CE, Dou SJ, El Sahly H, et al. Molecular genetic analysis of nucleotide polymorphisms associated with ethambutol resistance in human isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2000; 44:326–336. PMID: 10639358.
12. Almeida Da Silva PE, Palomino JC. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: classical and new drugs. J Antimicrob Chemother. 2011; 66:1417–1430. PMID: 21558086.
13. Zhang Y, Yew WW. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2009; 13:1320–1330. PMID: 19861002.
14. Morgan M, Kalantri S, Flores L, Pai M. A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2005; 5:62. PMID: 16050959.

15. Hillemann D, Rüsch-Gerdes S, Richter E. Evaluation of the GenoType MTBDRplus assay for rifampin and isoniazid susceptibility testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol. 2007; 45:2635–2640. PMID: 17537937.
16. Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralized use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011; 377:1495–1505. PMID: 21507477.
17. Chen J, Iannone MA, Li MS, Taylor JD, Rivers P, Nelsen AJ, et al. A microsphere-based assay for multiplexed single nucleotide polymorphism analysis using single base chain extension. Genome Res. 2000; 10:549–557. PMID: 10779497.

18. Armstrong B, Stewart M, Mazumder A. Suspension arrays for high throughput, multiplexed single nucleotide polymorphism genotyping. Cytometry. 2000; 40:102–108. PMID: 10805929.

19. Kellar KL, Iannone MA. Multiplexed microsphere-based flow cytometric assays. Exp Hematol. 2002; 30:1227–1237. PMID: 12423675.

20. Nolan JP, Mandy FF. Suspension array technology: new tools for gene and protein analysis. Cell Mol Biol (Noisy-le-grand). 2001; 47:1241–1256. PMID: 11838973.
21. Kim SJ, Bai GH, Hong YP. Drug-resistant tuberculosis in Korea, 1994. Int J Tuberc Lung Dis. 1997; 1:302–308. PMID: 9432384.
22. Dunbar SA. Application of Luminex xMAP technology for rapid, high-throughput multiplexed nucleic acid detection. Clin Chim Acta. 2006; 363:71–82. PMID: 16102740.
23. Lee SH, Walker DR, Creqan PB, Boerma HR. Comparison of four flow cytometric SNP detection assays and their use in plant improvement. Theor Appl Genet. 2004; 110:167–174. PMID: 15551036.

24. Bergval I, Sengstake S, Brankova N, Levterova V, Abadía V, Tadumaze N, et al. Combined species identification, genotyping, and drug resistance detection of Mycobacterium tuberculosis cultures by MLPA on a bead-based array. PLoS One. 2012; 7:e43240. PMID: 22916230.
25. Gomgnimbou MK, Hernández-Neuta I, Panaiotov S, Bachiyska E, Palomino JC, Martin A, et al. Tuberculosis-spoligo-rifampin-isoniazid typing: an all-in-one assay technique for surveillance and control of multidrug-resistant tuberculosis on Luminex devices. J Clin Microbiol. 2013; 51:3527–3534. PMID: 23966495.

26. Bortolin S, Black M, Modi H, Boszko I, Kobler D, Fieldhouse D, et al. Analytical validation of the tag-it high-throughput microsphere-based universal array genotyping platform: application to the multiplex detection of a panel of thrombophilia-associated single-nucleotide polymorphisms. Clin Chem. 2004; 50:2028–2036. PMID: 15364887.

Fig. 1
Sequencing chromatograms for indeterminate results of TAG-ASPE assay. It is not shown the sequencing chromatograms for inhA position _8 T→G and embB codon 531 TCG→ACG.
Abbreviation: ASPE, allele-specific primer extension.
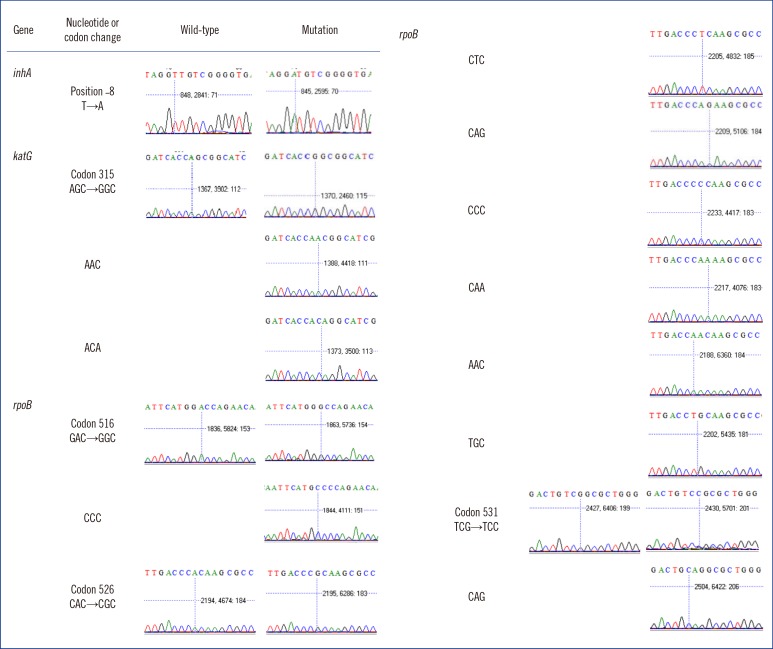
Table 1
Multiplex PCR primer sequences
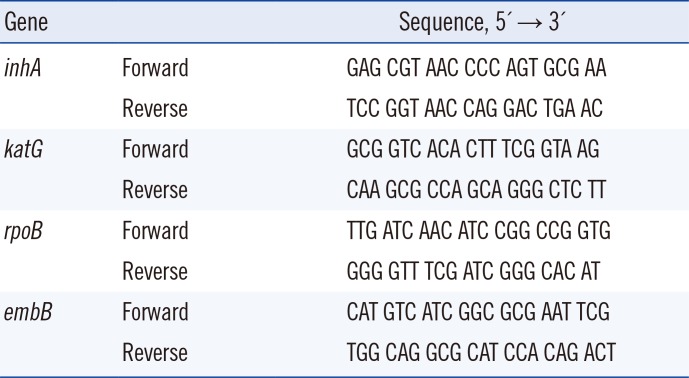
Table 2
TAG-ASPE primer sequences

Table 3
Mutations detected by sequencing and the TAG-ASPE method
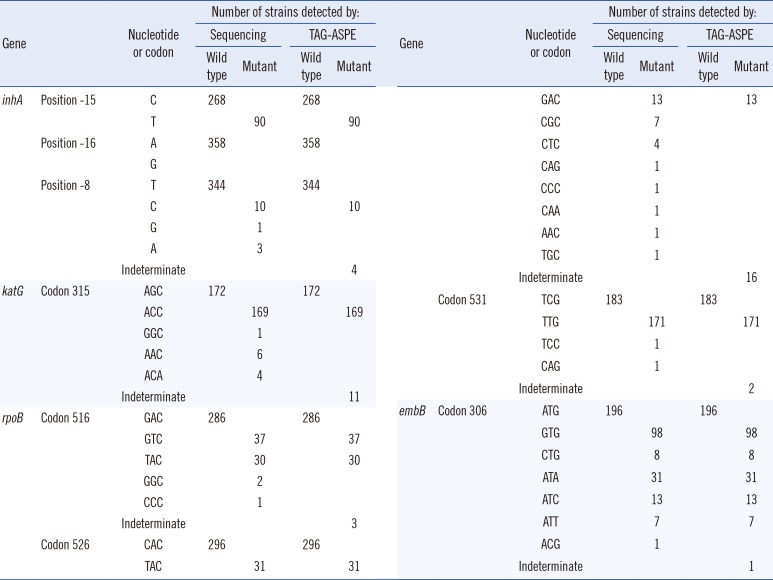
Table 4
Drug susceptibility testing by phenotypic DST and TAG-ASPE method




 PDF
PDF ePub
ePub Citation
Citation Print
Print


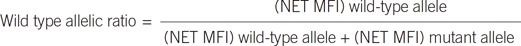

 XML Download
XML Download