Abstract
Background
Periodic monitoring of antimicrobial resistance trends of clinically important anaerobic bacteria such as Bacteroides fragilis group organisms is required. We determined the antimicrobial susceptibilities of clinical isolates of B. fragilis group organisms recovered from 2009 to 2012 in a tertiary-care hospital in Korea.
Methods
A total of 180 nonduplicate clinical isolates of B. fragilis group organisms were collected in a tertiary care hospital. The species were identified by conventional methods: the ATB 32A rapid identification system (bioMérieux, France) and the Vitek MS matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (bioMérieux). Antimicrobial susceptibility was determined by the CLSI agar dilution method.
Results
Imipenem and meropenem resistance rates were 0-6% for B. fragilis group isolates. The rate of resistance to piperacillin-tazobactam was 2% for B. fragilis and 0% for other Bacteroides species, but 17% for B. thetaiotaomicron isolates. High resistance rates to piperacillin (72% and 69%), cefotetan (89% and 58%), and clindamycin (83% and 69%) were observed for B. thetaiotaomicron and other Bacteroides spp. The moxifloxacin resistance rate was 27% for other Bacteroides spp. The MIC50 and MIC90 of tigecycline were 2-4 µg/mL and 8-16 µg/mL, respectively. No isolates were resistant to chloramphenicol or metronidazole.
Bacteroides fragilis group organisms are important anaerobic pathogens that frequently cause various infections such as intra-abdominal infection, postoperative wound infection, and bacteremia in humans [1, 2, 3, 4]. These organisms are the most antibiotic-resistant among the anaerobic isolates and are responsible for high rates of morbidity and mortality [4, 5, 6].
According to the CLSI guidelines, routine susceptibility testing may not be necessary for many individual patient isolates [7, 8, 9], because predicting and testing the susceptibility of anaerobes is technically difficult and time-consuming. However, antimicrobial resistance of B. fragilis group organisms varies by geographic location and species [1, 2, 5, 7, 10]. Furthermore, antimicrobial resistance of these organisms has consistently increased over the past few decades, and their susceptibility to antimicrobial agents has become less predictable. Therefore, periodic and local surveillance studies are considered necessary, and current susceptibility data are important for empirical antimicrobial therapy.
Most susceptibility studies use the CLSI methodology. However, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) publishes its own breakpoints, not all of which are equivalent to those of the CLSI [11]. Therefore, resistance rates may differ depending on the breakpoint used.
In this study, we determined the current susceptibilities of clinical isolates of the B. fragilis group organisms recovered in a tertiary-care hospital in Korea from 2009 to 2012, and we compared the resistant rates using both the CLSI and EUCAST breakpoints.
B. fragilis group organisms were isolated from blood, normally sterile body fluid, and abscess specimens in a university hospital in Korea between 2009 and 2012. All isolates were identified by conventional methods, a commercial rapid identification kit (ATB 32A, ANC; bioMérieux, Marcy I'Etoile, France) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; Vitek MS, bioMérieux). A total of 180 nonduplicate isolates were used in this study, including 86 B. fragilis, 46 B. thetaiotaomicron, 20 Bacteroides vulgatus, 13 Bacteroides ovatus, 13 Parabacteroides distasonis and 2 Bacteroides uniformis isolates.
Antimicrobial susceptibility was determined by the CLSI agar dilution method [9]. The medium used was Brucella agar (Becton Dickinson, Cockeysville, MD, USA) supplemented with 5 µg/mL hemin, 1 µg/mL vitamin K1, and 5% laked sheep blood. The antimicrobials used were piperacillin and tazobactam (Yuhan, Seoul, Korea), cefoxitin (Merck Sharp & Dohme, West Point, PA, USA), cefotetan (Daiichi Pharmaceutical, Tokyo, Japan), clindamycin (Korea Upjohn, Seoul, Korea), imipenem and metronidazole (ChoongWae, Seoul, Korea), chloramphenicol (Chong Kun Dang, Seoul, Korea), meropenem (Sumitomo, Tokyo, Japan), moxifloxacin (Bayer Korea, Seoul, Korea) and tigecycline (Wyeth Research, Pearl River, NY, USA); they were used in the powder form.
For piperacillin-tazobactam, serial two-fold dilutions of piperacillin were combined with tazobactam at constant concentrations of 4 µg/mL.
The plates were inoculated with 105 colony-forming unit (CFU) with a Steers replicator (Craft Machine Inc., Woodline, PA, USA) and incubated in an anaerobic chamber (Forma Scientific, Marietta, OH, USA) for 48 hr at 37℃. The minimum inhibitory concentration (MIC) was defined as the lowest antibiotic concentration that caused a marked reduction in growth, such as from confluent growth to a haze, less than 10 tiny colonies, or several normal-sized colonies [9]. B. fragilis ATCC 25285 and B. thetaiotaomicron ATCC 29741 were used as controls. The MICs were interpreted using the breakpoints recommended by CLSI and EUCAST for anaerobic bacteria [9, 13]. Since neither CLSI nor EUCAST recommends breakpoints for tigecycline, the breakpoints recommended by the US Food and Drug Administration (FDA), ≤4 and ≥16 µg/mL, were used [12].
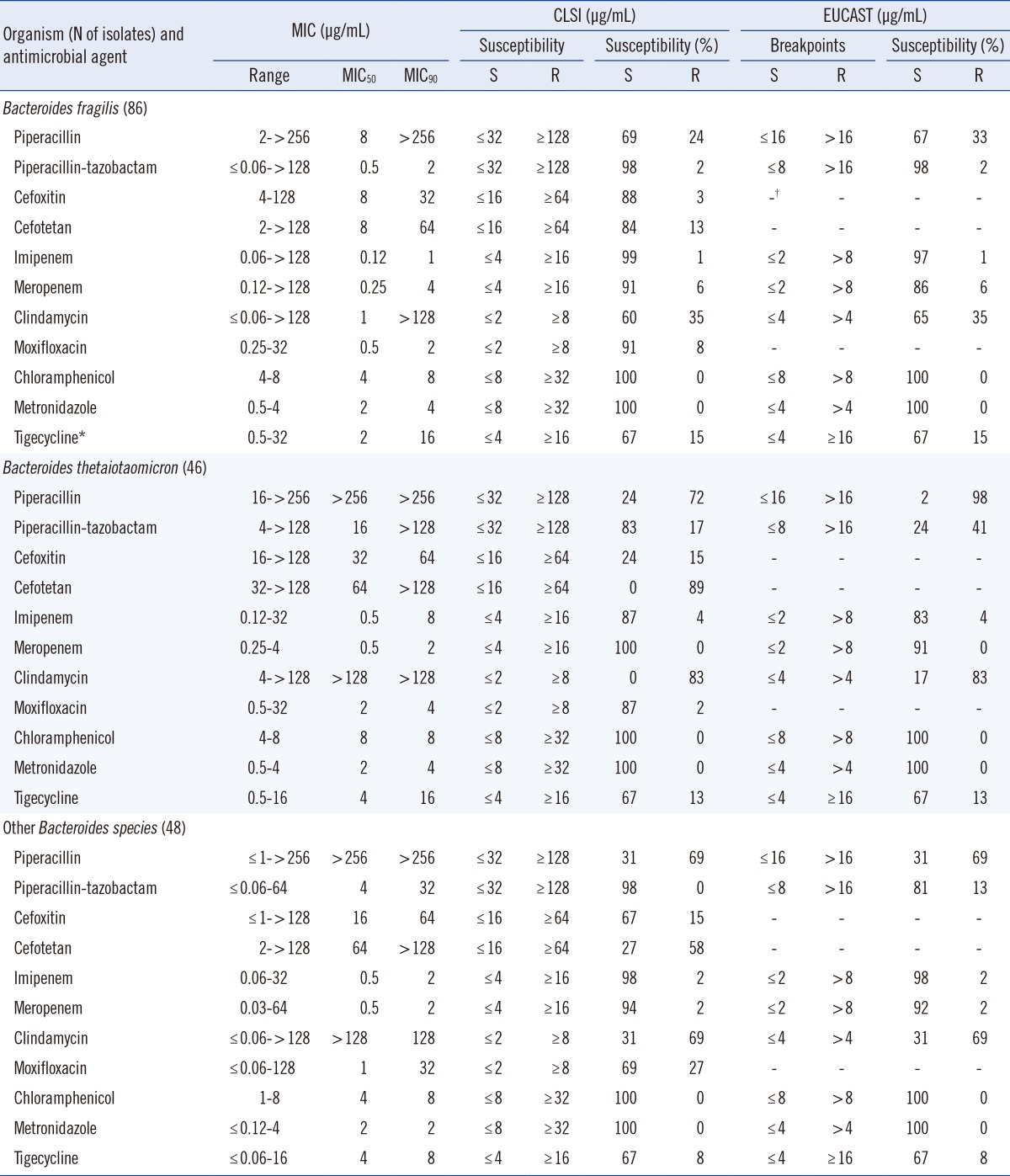
MIC ranges, MIC50s, MIC90s, and the percentages of resistant isolates for various antimicrobial agents are shown in Table 1. Imipenem resistance rates were less than 5% for B. fragilis group isolates. There were four imipenem-resistant isolates: one B. fragilis, two B. thetaiotaomicron, and one B. ovatus. The resistance rates to meropenem were 0-6% for all tested B. fragilis group organisms. High resistance rates to piperacillin were observed (72% and 69%) for B. thetaiotaomicron isolates and other Bacteroides spp., respectively. The rate of resistance to piperacillin-tazobactam was 2% for B. fragilis, 0% for other Bacteroides spp., and 17% for B. thetaiotaomicron isolates. Cefoxitin is an active β-lactam drug used against B. fragilis group organisms, but our results showed an increase in resistance rates to this drug. The rates of resistance to cefotetan (89% and 58%) increased prominently for B. thetaiotaomicron isolates and other Bacteroides spp., respectively. The resistance rates for clindamycin were 83% and 69% for B. thetaiotaomicron and other Bacteroides spp., respectively. The resistance rates for moxifloxacin were 8%, 2%, and 27% for B. fragilis, B. thetaiotaomicron, and other Bacteroides spp., respectively. The MIC range for tigecycline was ≤0.06-32 µg/mL for all B. fragilis group isolates. The resistance rates of tigecycline were 15%, 13%, and 8% for B. fragilis, B. thetaiotaomicron, and other Bacteroides spp., respectively.
All the isolates were inhibited by ≤8 µg/mL chloramphenicol or metronidazole, to which no isolates were resistant.
The EUCAST breakpoints were equal to or lower than the CLSI breakpoints for most antibiotics tested in this study. Large differences in resistance rates when analyzed with CLSI or EUCAST breakpoints were observed for piperacillin: 24% vs. 33% for B. fragilis and 72% vs. 98% for B. thetaiotaomicron, respectively. The piperacillin-tazobactam resistance rates were 17% vs. 41% for B. thetaiotaomicron and 0% vs. 13% for other Bacteroides species by CLSI or EUCAST breakpoints, respectively. No other resistance rates differed by CLSI or EUCAST.
Susceptibility testing had not been routinely used for treating infections involving anaerobes because of the availability of broad-spectrum antibiotics, delayed reporting due to slow growth of bacteria, and the assumption that anaerobe susceptibility patterns do not change [13, 14]. However, antimicrobial susceptibility testing is indispensable in patients with serious or life-threatening infections, because the clinical outcome correlates with the test results [14, 15]. In addition, an increasing resistance of anaerobes to several antibiotics has recently been reported [3, 7, 11].
Carbapenems are usually highly active against B. fragilis group isolates. A study performed from 1989 to 1990 found no carbapenem-resistant B. fragilis group isolates [16]. Although the level of carbapenem resistance has not changed markedly during the past 25 yr, the percentage of isolates with reduced susceptibilities has steadily increased. In this study, the meropenem resistance rate was 6% for B. fragilis isolates. This rate is higher than the approximately 1% rate reported for B. fragilis in Europe, USA, and Canada, but lower than the rate (7.5%) reported in Germany by Seifert et al. [10]. Piperacillin resistance increased to 72% and 69% for B. thetaiotaomicron and other Bacteroides group spp., respectively, which are higher than the rates reported 10 yr ago (42% and 49%, respectively). A study performed from 1997 to 2004 reported the MIC90 and resistance rate to piperacillin-tazobactam to be 16 µg/mL and 4% [6]; in this study, they were found to increase to >128 µg/mL and 17%, respectively, for B. thetaiotaomicron. These rates were slightly higher than that reported for piperacillin-tazobactam in a multicenter survey of 13 European countries; the reported MIC90 was 128 µg/mL and the resistance rate was 12% [3]. Other countries, such as the USA, Canada, and Argentina, have reported relatively low piperacillin-tazobactam resistance rates (<4%) for all B. fragilis group isolates [2, 7, 14]. Compared to cefoxitin, cefotetan is less effective against B. fragilis group isolates. Recently, the Infectious Diseases Society of America (IDSA) removed cefotetan from its list of recommended therapies for intra-abdominal infections because it has poor activity against the B. fragilis group and results in clinical failures [11]. The present study also reported a poor activity of cefotetan; the resistance rates were 89% for B. thetaiotaomicron and 58% for other B. fragilis group organisms.
Moxifloxacin had good in vitro activity against most anaerobic bacteria when first introduced [15]; however, various recent surveys have reported increased resistance among Bacteroides spp. [2, 3, 10, 14, 16, 17]. Our previous studies also reported increased resistance to moxifloxacin among Bacteroides species and found a resistance rate of 11-20% in B. fragilis [8, 17]. However, the present data for moxifloxacin show low resistance (8% and 2%, respectively) for B. fragilis and B. thetaiotaomicron and a relatively high resistance rate (27%) in other Bacteroides species (Table 1).
Tigecycline has been approved by the FDA for use in complicated skin and soft tissue infections and intra-abdominal infections. Despite expectations, tigecycline resistance rates were 8-15% for B. fragilis group species in this study. Some studies have shown variable resistance rates, such as 0-10% in Europe and Argentina [3, 7] and 0-17.5% in Canada [2]. In addition, this study found a relatively high percentage of intermediate rates (17-25% for all B. fragilis group isolates); therefore, continuous attention is required.
Metronidazole and chloramphenicol resistance in B. fragilis group isolates have recently been reported worldwide [3, 10, 15, 18, 19]. Resistance to metronidazole was recently reported to be <1% in Europe and the USA [3, 14], but it has been approaching 1% in many European countries [10]. However, no B. fragilis group isolates that are resistant to these agents have been reported previously in Korea. Consistent with previous reports [6, 8, 17, 20], susceptibility patterns for metronidazole and chloramphenicol remained stable.
To compare the differences between using CLSI and EUCAST clinical breakpoints, the available EUCAST breakpoints were also used. EUCAST breakpoints were not available for cefoxitin, cefotetan, moxifloxacin, and tigecycline. EUCAST stated that there is insufficient evidence that anaerobes are a good target for therapy with moxifloxacin [13]. They also stated that there is clinical evidence of tigecycline activity in mixed intra-abdominal infections, but no correlation between MIC values and clinical outcomes; therefore, no breakpoints were given [13]. The most prominent difference between CLSI and EUCAST is the higher CLSI breakpoint for piperacillin and piperacillin-tazobactam. We expected higher resistance rates according to the EUCAST breakpoints; however, only the piperacillin and piperacillin-tazobactam resistance patterns differed significantly.
In conclusion, imipenem, meropenem, chloramphenicol, and metronidazole remain active against B. fragilis group isolates. Moxifloxacin resistance rates were 8%, 2%, and 27% for B. fragilis, B. thetaiotaomicron, and other Bacteroides spp., respectively. Tigecycline resistance rates for B. fragilis group species were 8-15%. Therefore, periodic monitoring is needed to demonstrate changes in the antimicrobial susceptibility patterns of B. fragilis group isolates.
Acknowledgments
This study was supported by a CMB-Yuhan research grant of Yonsei University College of Medicine for 2012(6-2012-0048).
References
1. Treviño M, Areses P, Peñalver MD, Cortizo S, Pardo F, del Molino ML, et al. Susceptibility trends of Bacteroides fragilis group and characterisation of carbapenemase-producing strains by automated REP-PCR and MALDI TOF. Anaerobe. 2012; 18:37–43. PMID: 22261518.
2. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2012; 56:1247–1252. PMID: 22203594.
3. Nagy E, Urbán E, Nord CE. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect. 2011; 17:371–379. PMID: 20456453.
4. Fille M, Mango M, Lechner M, Schaumann R. Bacteroides fragilis group: trends in resistance. Curr Microbiol. 2006; 52:153–157. PMID: 16450067.
5. Snydman DR, Jacobus NV, McDermott LA, Ruthazer R, Golan Y, Goldstein EJ, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007; 51:1649–1655. PMID: 17283189.
6. Roh K, Kim S, Kim CK, Yum JH, Kim MS, Yong D, et al. Resistance trends of Bacteroides fragilis group over an 8-year period, 1997-2004, in Korea. Korean J Lab Med. 2009; 29:293–298. PMID: 19726890.
7. Fernández-Canigia L, Litterio M, Legaria MC, Castello L, Predari SC, Di Martino A, et al. First national survey of antibiotic susceptibility of the Bacteroides fragilis group: emerging resistance to carbapenems in Argentina. Antimicrob Agents Chemother. 2012; 56:1309–1314. PMID: 22232282.
8. Lee Y, Park Y, Kim MS, Yong D, Jeong SH, Lee K, et al. Antimicrobial susceptibility patterns for recent clinical isolates of anaerobic bacteria in South Korea. Antimicrob Agents Chemother. 2010; 54:3993–3997. PMID: 20585132.

9. CLSI. Methods for Antimicrobial susceptibility testing of anaerobic bacteria; Approved standard, 8th ed. CLSI document M11-A8. Wayne, PA: Clinical and Laboratory Standards Institute;2012.
10. Seifert H, Dalhoff A. German multicentre survey of the antibiotic susceptibility of Bacteroides fragilis group and Prevotella species isolated from intra-abdominal infections: results from the PRISMA study. J Antimicrob Chemother. 2010; 65:2405–2410. PMID: 20851813.
11. Brook I, Wexler HM, Goldstein EJ. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev. 2013; 26:526–546. PMID: 23824372.

12. The US Food and Drug Administration. FDA Approved Drug Products Label for TYGACIL, NDA no. 021821 (approved on 23/05/2013). http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021821s037lbl.pdf.
13. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 3.1, 2013. http://www.eucast.org/clinical_breakpoints/.
14. Snydman DR, Jacobus NV, McDermott LA, Golan Y, Hecht DW, Goldstein EJ, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis. 2010; 50(Sl):S26–S33. PMID: 20067390.

15. Goldstein EJC. Resistance trends in antimicrobial susceptibility of anaerobic bacteria, part II. Clinical Microbiology Newsletter. 2011; 33:9–15.

16. Betriu C, Rodríguez-Avial I, Gómez M, Culebras E, Picazo JJ. Changing patterns of fluoroquinolone resistance among Bacteroides fragilis group organisms over a 6-year period (1997-2002). Diagn Microbiol Infect Dis. 2005; 53:221–223. PMID: 16243476.
17. Cho S, Chung HS, Lee Y, Kim M, Yong D, Jeong SH, et al. In vitro activities of ceftriaxone-sulbactam against major aerobic and anaerobic bacteria from clinical samples. Lab Med Online. 2011; 1:209–220.

18. Wybo I, Van den Bossche D, Soetens O, Vekens E, Vandoorslaer K, Claeys G, et al. Fourth Belgian multicentre survey of antibiotic susceptibility of anaerobic bacteria. J Antimicrob Chemother. 2014; 69:155–161. PMID: 24008826.

19. Brazier JS, Stubbs SL, Duerden BI. Metronidazole resistance among clinical isolates belonging to the Bacteroides fragilis group: time to be concerned. J Antimicrob Chemother. 1999; 44:580–581. PMID: 10588328.
20. Lee K, Shin HB, Chong Y. Antimicrobial resistance patterns of Bacteroides fragilis group organisms in Korea. Yonsei Med J. 1998; 39:578–586. PMID: 10097686.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download