Abstract
Background
Antimicrobial susceptibility testing (AST) of Clostridium difficile is increasingly important because of the rise in resistant strains. The standard medium for the AST of C. difficile is supplemented Brucella agar (sBA), but we found that the growth of C. difficile on sBA was not optimal. Because active growth is critical for reliable AST, we developed a new, modified C. difficile (mCD) agar. C. difficile grew better on mCD agar than on sBA.
Methods
C. difficile isolates were collected from patients with healthcare-associated diarrhea. sBA medium was prepared according to the CLSI guidelines. Homemade mCD agar containing taurocholate, L-cysteine hydrochloride, and 7% horse blood was used. For 171 C. difficile isolates, we compared the agar dilution AST results from mCD agar with those from sBA.
Results
No significant differences were observed in the 50% minimal inhibitory concentration (MIC50) and 90% minimal inhibitory concentration (MIC90) of clindamycin (CLI), metronidazole (MTZ), moxifloxacin (MXF), piperacillin-tazobactam (PTZ), and rifaximin (RIX), but the values for vancomycin (VAN) were two-fold higher on mCD agar than on sBA. The MICs of CLI, MXF, and RIX were in 100% agreement within two-fold dilutions, but for MTZ, VAN, and PTZ, 13.7%, 0.6%, and 3.1% of the isolates, respectively, were outside the acceptable range.
Clostridium difficile is the most common cause of antibiotic-associated diarrhea. As the name implies, culture of this anaerobic pathogen is sometimes difficult, and the likelihood of isolation can be affected by factors, such as the quality of the anaerobic conditions, specimen treatment method, skill of personnel, and culture media [1, 2]. Active growth is especially critical in situations where rapid and confluent growth of a microorganism is essential, as it is for antimicrobial susceptibility testing (AST).
The first-line antimicrobial for the treatment of mild C. difficile-associated diarrhea (CDAD) has been metronidazole (MTZ), but for severe CDAD, vancomycin (VAN) is recommended as the first-line agent [3]. The recent emergence of hypervirulent C. difficile strains associated with high relapse and mortality rates [4, 5] calls for AST for epidemiologic purposes and in individual cases of treatment failure. Occasional reports of MTZ- or VAN-resistant C. difficile isolates [6, 7] and the increase in non-susceptible isolates also support the need for AST of this anaerobic organism.
The anaerobe working group of the CLSI recommends the agar dilution method with supplemented Brucella agar (sBA) as the reference method for C. difficile AST and the gradient method (Etest) for clinical laboratories [8]. The addition of sodium taurocholate to the culture medium has been reported to increase the recovery of C. difficile from stool specimens [9]. Cysteine hydrochloride has also been used as a growth supplement for the isolation of C. difficile [10]. We recently had trouble in subculturing deep-frozen C. difficile strains on commercially prepared C. difficile selective agar (BBL Clostridium difficile Selective Agar; BD Diagnostics, Sparks, MD, USA). Therefore, we modified the C. difficile agars by adding 0.1% taurocholate and 0.05% cysteine hydrochloride and found that the C. difficile colonies grew larger and faster on the modified C. difficile (mCD) agar than on the prepared C. difficile agar. We extended our pilot study to sBA, which is the standard medium for AST of C. difficile according to CLSI guidelines. The interpretation of Etest results was easier with mCD agar than with sBA, because growth on mCD agar was more confluent than on sBA. To apply our preliminary observations on the standard agar dilution method for C. difficile, the AST results from mCD agar were compared with those from the CLSI-recommended sBA for 171 clinical isolates of C. difficile.
Stool specimens were collected from patients with healthcare-associated diarrhea admitted to Hanyang University Guri Hospital in Guri, South Korea, from September 2008 to January 2010. After alcohol shock treatment, specimens were inoculated on Clostridium difficile Selective Agar (Oxoid Ltd., Cambridge, UK) supplemented with cycloserine, cefoxitin, and 7% horse blood. Specimens were cultured anaerobically for 72 hr. Colonies of C. difficile were identified with an API RapidID 32A system (bioMérieux SA, Lyon, France), and toxigenic isolates were stored in a -70℃ deep freezer. A total of 171 C. difficile isolates were collected and used for the study. This study was approved by the institutional review board of Hanyang University Guri Hospital (HYUGH IRB 2013-24).
sBA (BBL Brucella Agar, Dehydrated; BD Diagnostics) containing hemin (5 µg/mL), vitamin K1 (10 µg/mL), and 5% sheep blood is recommended for the AST of C. difficile by the CLSI. Homemade mCD agar (Clostridium difficile Agar Base; Oxoid Ltd., Hampshire, England) containing taurocholate (0.1%), L-cysteine hydrochloride (0.05%; Sigma-Aldrich, Buchs SF, Switzerland), and 7% horse blood was compared to sBA for the AST of the 171 C. difficile isolates.
The six antimicrobial agents used in the AST were MTZ, VAN, clindamycin (CLI), moxifloxacin (MXF), piperacillin-tazobactam (PTZ), and rifaximin (RIX). Five of the antibiotics (MTZ, VAN, MXF, PTZ, and RIX) were added to sBA and mCD agar to make AST agar plates containing various concentrations of antibiotics.
After thawing, stock strains were subcultured twice and suspended in saline to 1 McFarland turbidity unit. The minimum inhibitory concentrations (MICs) of the antibiotics were determined by the agar dilution method for the AST of anaerobic bacteria suggested by CLSI [8]. However, the MIC of CLI was determined by Etest [11], because the CLI AST was added after completing the ASTs for MTZ, VAN, MXF, PTZ, and RIX.
Bacterial suspensions were inoculated with a 36-pin replicator onto sBA and mCD agar containing various concentrations of antimicrobials. After incubation in an anaerobic chamber (SHEL LAB Bactron Anaerobic Chamber; Sheldon Manufacturing Inc., Cornelius, OR, USA) at 37℃ for 48 hr, we determined the MICs of C. difficile isolates according to the CLSI guidelines. C. difficile ATCC 700057 was used as a QC strain for each experiment, and the results were accepted only when the QC results were within an acceptable range. The MICs of CLI were determined by Etest (bioMérieux, Craponne, France) with the same media and inocula.
Results were analyzed by using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA), and two-sample t-tests were used to compare the geometric means (geomean) of the MIC values. The geomean for an MIC is a transformed datum derived by using logarithms to generate a normal distribution [12]. Statistical significance was set at P<0.05. A very major error was recorded when an isolate was susceptible on mCD agar and resistant on sBA (false susceptible), and a major error was recorded when an isolate was susceptible on sBA but resistant on mCD agar (false resistant).
Fig. 1 presents the MIC distributions of the six antimicrobial agents against the 171 clinical isolates of C. difficile on sBA and mCD agar. The MIC distributions of MTZ, VAN, and PTZ were unimodal, whereas those of CLI, MXF, and RIX were bimodal (Fig. 1).
There were no significant differences in the MIC50 and MIC90 of MTZ, CLI, MXF, and PTZ on sBA versus mCD agar, but the MIC50 and MIC90 for VAN were two-fold higher on mCD agar (Table 1). A two-fold difference in the MIC for VAN was considered within the acceptable range for QC strains in agar dilution tests according to the CLSI standard M11-A8.
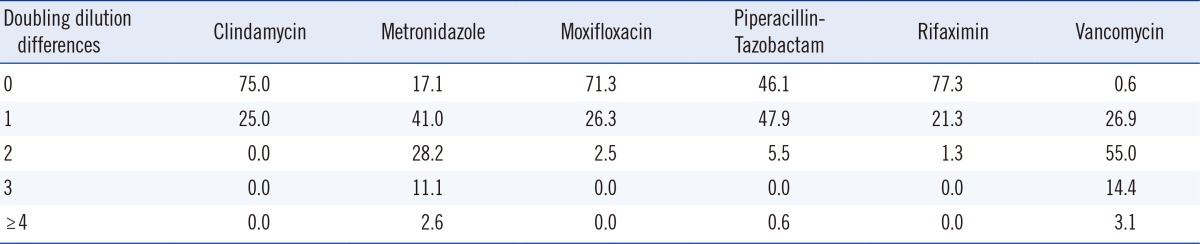
The percent agreement of the MIC results for the six antimicrobials on sBA and mCD agar is shown in Table 2. The two-fold dilution MICs for CLI, MXF, and RIX were in 100% agreement, but 13.7%, 0.6%, and 3.1% of the isolates were outside the acceptable range for MTZ, VAN, and PTZ, respectively. The geomean MICs for VAN and PTZ were significantly higher on mCD agar than on sBA (P<0.001), but the MIC of MTZ was higher on sBA (P=0.0089) (Table 1).
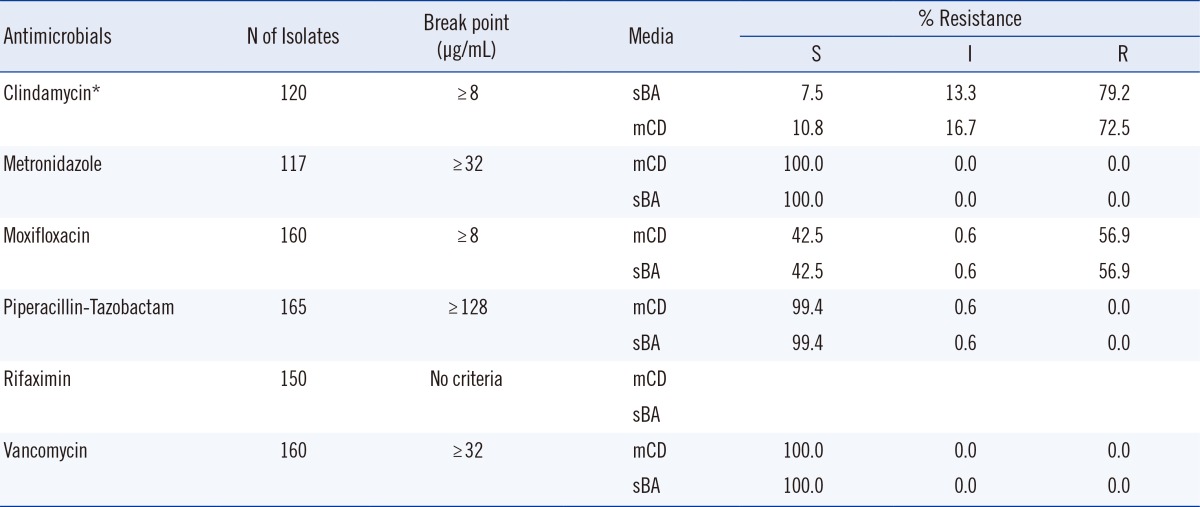
The percent resistance of the 171 C. difficile isolates to the antimicrobials is shown in Table 3. The rates of resistance to CLI and MXF on sBA were 79.2% and 56.9%, respectively, and the rates on mCD agar were not significantly different. No MTZ, VAN, or PTZ resistance was detected on sBA by the CLSI breakpoint criteria, but according to the recent European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (breakpoints: >2 mg/L for MTZ and VAN), 0.9% and 1.9% of the strains were resistant to MTZ and VAN, respectively.
There were no very major errors or major errors for any of the antimicrobials (MTZ, VAN, CLI, MXF, and PTZ), with the exception of RIX, for which there are currently no criteria for resistance breakpoints.
To identify conditions that promote the active growth of C. difficile, which is important for AST, we compared the AST results from mCD agar with those from the CLSI-recommended sBA for 171 clinical isolates of C. difficile. The MIC distributions for the 171 C. difficile isolates differed depending on the antimicrobial agents (Fig. 1). For MTZ, VAN, and PTZ, unimodal distributions with relatively narrow ranges of MIC distributions were observed. However, the MIC distributions for CLI, MXF, and RIX were bimodal or skewed and divided in two by the breakpoints for each antibiotic. Our results with sBA were comparable with the EUCAST data. Some of the CLI MIC data clustered on ≥256 µg/mL, and some RIX MICs clustered on ≥8 µg/mL. The VAN MIC distribution on mCD agar was shifted to the right compared to the distribution on sBA. The shift to the right on mCD agar was anticipated from the outset, because growth was more active on mCD agar. However, for VAN and PTZ, this expectation was confirmed only by the geomean data, not by the MIC50 or MIC90 data.
Using five hypothetical series of MIC values, Davis [13] demonstrated that the MIC50, MIC75, and MIC90 values were identical for five antimicrobials. However, their geomean MIC values varied from 1.80 to 5.86. The ranges of the MIC, MIC50, MIC90, and geomean for each antibiotic on mCD agar and sBA are shown in Table 1. The differences between the MIC50 and MIC90 on the two media were all within the acceptable range (±two-fold dilutions). However, the geomean MICs of VAN and PTZ were significantly above the acceptable range on mCD medium (both P<0.0001), but the geomean MIC of MTZ was below the acceptable range (P=0.0089). The geomean of CLI, MXF, or RIX was not significantly different on the two media. Cumulative percentages, such as MIC50 and MIC90, are suitable for comparing AST media or antibiotic potency for unimodal MIC distributions, but not suitable for clear-cut bimodal MIC distributions [14]. However, our analysis showed that the geomean MIC might be more powerful for evaluating AST methods or antibiotic potency than cumulative percentages, for both unimodal and bimodal MIC distributions.
The MIC50/MIC90 of MTZ and VAN on sBA were 1/2 µg/mL and 1/2 µg/mL, respectively, similar to the data reported in the other Korean studies (1/4 and 0.5/1 µg/mL) [15]. Our results for MTZ and VAN were also within two-fold of the results obtained by Huang et al. [16] in China (0.125/0.25 and 0.5/1 µg/mL), Hecht et al. [17] in USA (0.125/0.25 and 1/1 µg/mL), and EUCAST (0.25/0.5 and 0.5/1 µg/mL) [18]. The MIC50/MIC90 of PTZ in our study were 16/16 µg/mL, similar to the values reported by Kim et al. [15] and Huang et al. [19] (8/16 µg/mL), but higher than the EUCAST values (4/8 µg/mL).
The percent agreement of the MIC values for the six antimicrobials on sBA and mCD agar is shown in Table 2. The MICs for CLI, MXF, and RIX were in 100% agreement within two-fold dilutions, but 13.7%, 0.6%, and 3.1% of the values for MTZ, PTZ, and VAN, respectively, were outside the acceptable range. The acceptable ranges of the MICs for the control strain (C. difficile ATCC 700057) according to the CLSI guideline are two-fold dilutions, except for VAN, for which three-fold dilutions are acceptable. When we applied this criterion, MTZ had a percentage higher than the acceptable MIC range. MTZ has been reported to be sensitive to light and low and high temperatures [20,21,22]. Similar poor reproducibility of MICs was also observed in susceptibility testing of MTZ against Helicobacter pylori [23]. We suspect that the exceptional AST results for MTZ were due to the instability of MTZ itself, but the mechanism needs to be verified.
The percent resistances of the 171 C. difficile isolates are displayed in Table 3 and Fig. 1. The percent resistances on the two media did not differ significantly. Resistance rates to CLI and MXF using sBA were quite high, and the percent resistances on mCD agar were similar. On sBA, no MTZ, VAN, or PTZ resistance was detected by the CLSI criteria, but according to the recent EUCAST guidelines (epidemiological breakpoints: >2 mg/L for MTZ and VAN), 0.9% and 1.9% of the isolates were resistant to MTZ and VAN, respectively. The review by Huang et al. [7] on antimicrobial resistance in C. difficile described recent reports on the emergence of MTZ-resistant strains around the globe. Pelaez et al. [6] have described the antimicrobial susceptibilities of 415 C. difficile isolates to MTZ and VAN over an 8-yr period (1993 to 2000) in Spain. The resistance rate to MTZ at the critical breakpoint (16 µg/mL) was 6.3%. Although full resistance to VAN was not observed, the rate of intermediate resistance was 3.1%. The emergence of reduced susceptibility to MTZ was recently reported in C. difficile isolates in UK [24]. The MIC for the historical C. difficile ribotype 001 was 1.03 mg/L, compared with 5.94 mg/L (P<0.001) for the recent isolates with reduced MTZ susceptibility (24.4% of isolates).
Very major errors and major errors were not found for any of the antimicrobials (MTZ, VAN, CLI, MXF, and PTZ), with the exception of RIX, for which no criteria for breakpoints are presently available. The MIC50 of RIX (0.007 µg/mL on sBA) was similar to the values reported by Hecht et al. [17] (0.0075 µg/mL) and Jiang et al. [25] (<0.002 µg/mL), but the MIC90 (>8 µg/mL) in this study was higher than the values obtained in the previous studies (0.015 and 0.25 µg/mL, respectively). In evaluating new media for AST, percent resistance would not be useful in the absence of corresponding CLSI or EUCAST standard breakpoints.
Although C. difficile growth was faster and colony size was larger on mCD agar than on sBA, the MIC distributions of the six antibiotics were not significantly different, except for the MIC50 and MIC90 of VAN, which were two-fold higher on mCD agar. We concluded that the MIC ranges of the MIC50 and MIC90 were acceptable for AST on both sBA and mCD agar, but the geomean MICs for MTZ, PTZ, and VAN were significantly different between the two media. We found that the geomean MIC had more differentiating power than the percent MIC distribution. Before using mCD agar as the medium for AST of C. difficile, more experiments are needed.
References
1. Marler LM, Siders JA, Wolters LC, Pettigrew Y, Skitt BL, Allen SD. Comparison of five cultural procedures for isolation of Clostridium difficile from stools. J Clin Microbiol. 1992; 30:514–516. PMID: 1537928.
2. Arroyo LG, Rousseau J, Willey BM, Low DE, Staempfli H, McGeer A, et al. Use of a selective enrichment broth to recover Clostridium difficile from stool swabs stored under different conditions. J Clin Microbiol. 2005; 43:5341–5343. PMID: 16208013.
3. Kelly CP, LaMont JT. Clostridium difficile-more difficult than ever. N Engl J Med. 2008; 359:1932–1940. PMID: 18971494.
4. Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005; 173:1037–1042. PMID: 16179431.
5. McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, Sambol SP, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005; 353:2433–2441. PMID: 16322603.
6. Peláez T, Alcalá L, Alonso R, Rodríguez-Créixems M, García-Lechuz JM, Bouza E. Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin. Antimicrob Agents Chemother. 2002; 46:1647–1650. PMID: 12019070.
7. Huang H, Weintraub A, Fang H, Nord CE. Antimicrobial resistance in Clostridium difficile. Int J Antimicrob Agents. 2009; 34:516–522. PMID: 19828299.
8. Clinical and Laboratory Standards Institute. M11-A7. Methods for animicrobialsusceptibilitytesting of anaerobic bacteria; Approved Standard. 7th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2007.
9. Bliss DZ, Johnson S, Clabots CR, Savik K, Gerding DN. Comparison of cycloserine-cefoxitin-fructose agar (CCFA) and taurocholate-CCFA for recovery of Clostridium difficile during surveillance of hospitalized patients. Diagn Microbiol Infect Dis. 1997; 29:1–4. PMID: 9350408.
10. Aspinall ST, Hutchinson DN. New selective medium for isolating Clostridium difficile from faeces. J Clin Pathol. 1992; 45:812–814. PMID: 1401214.
11. Erikstrup LT, Danielsen TK, Hall V, Olsen KE, Kristensen B, Kahlmeter G, et al. Antimicrobial susceptibility testing of Clostridium difficile using EUCAST epidemiological cut-off values and disk diffusion correlates. Clin Microbiol Infect. 2012; 18:E266–E272. PMID: 22672504.
12. Bland JM. AltmanDG. Statistics notes-Transforming data. Br Med J. 1996; 312:770. PMID: 8605469.
13. Davies BI. The importance of the geometric mean MIC. J Antimicrob Chemother. 1990; 25:471–472. PMID: 2338423.

14. Reynolds R, Hope R, Williams L. BSACWorking Parties on Resistance Surveillance. Survey, laboratory and statistical methods for the BSAC Resistance Surveillance Programmes. J Antimicrob Chemother. 2008; 62(S2):ii15–ii28. PMID: 18819976.
15. Kim H, Jeong SH, Roh KH, Hong SG, Kim JW, Shin MG, et al. Investigation of toxin gene diversity, molecular epidemiology, and antimicrobial resistance of Clostridium difficile isolated from 12 hospitals in South Korea. Korean J Lab Med. 2010; 30:491–497. PMID: 20890081.
16. Huang H, Wu S, Wang M, Zhang Y, Fang H, Palmgren AC, et al. Clostridium difficile infections in a Shanghai hospital: antimicrobial resistance, toxin profiles and ribotypes. Int J Antimicrob Agents. 2009; 33:339–342. PMID: 19097757.
17. Hecht DW, Galang MA, Sambol SP, Osmolski JR, Johnson S, Gerding DN. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob Agents Chemother. 2007; 51:2716–2719. PMID: 17517836.
18. European Committee on Antimicrobial Susceptibility. Breakpoint tables for interpretation of MICs and zone diameters. Version 3.0. Basel: EUCAST;2013.
19. Huang H, Weintraub A, Fang H, Wu S, Zhang Y, Nord CE. Antimicrobial susceptibility and heteroresistance in Chinese Clostridium difficile strains. Anaerobe. 2010; 16:633–635. PMID: 20849968.
20. Chen J, Jiang XY, Chen XQ, Chen Y. Effect of temperature on the metronidazole-BSA interaction: Multi-spectroscopic method. J Mol Struct. 2008; 876:121–126.

21. Zietsman S, Kilian G, Worthington M, Stubbs C. Formulation development and stability studies of aqueous metronidazole benzoate suspensions containing various suspending agents. Drug Dev Ind Pharm. 2007; 33:191–197. PMID: 17454051.

22. Wang DP, Yeh MK. Degradation kinetics of metronidazole in solution. J Pharm Sci. 1993; 82:95–98. PMID: 8429500.

23. Kang JO, Han D, Choi TY. Evaluation of four methods for antimicrobial susceptibility testing of Helicobacter pylori in routine practice. Korean J Clin Microbiol. 2005; 8:82–89.
24. Baines SD, O'Connor R, Freeman J, Fawley WN, Harmanus C, Mastrantonio P, et al. Emergence of reduced susceptibility to metronidazole in Clostridium difficile. J Antimicrob Chemother. 2008; 62:1046–1052. PMID: 18693234.
25. Jiang ZD, DuPont HL, La Rocco M, Garey KW. In vitro susceptibility of Clostridium difficile to rifaximin and rifampin in 359 consecutive isolates at a university hospital in Houston, Texas. J Clin Pathol. 2010; 63:355–358. PMID: 20354207.
Fig. 1
Distribution of the minimum inhibitory concentrations (MIC) (x-axis, units are µg/mL) of six antimicrobial agents against the 171 clinical isolates of Clostridium difficile on supplemented Brucella agar (gray bars) and modified CD agar (black bars). The dotted lines represent the breakpoints for the antimicrobials, with the exception of rifaximin, which does not presently have breakpoint criteria.
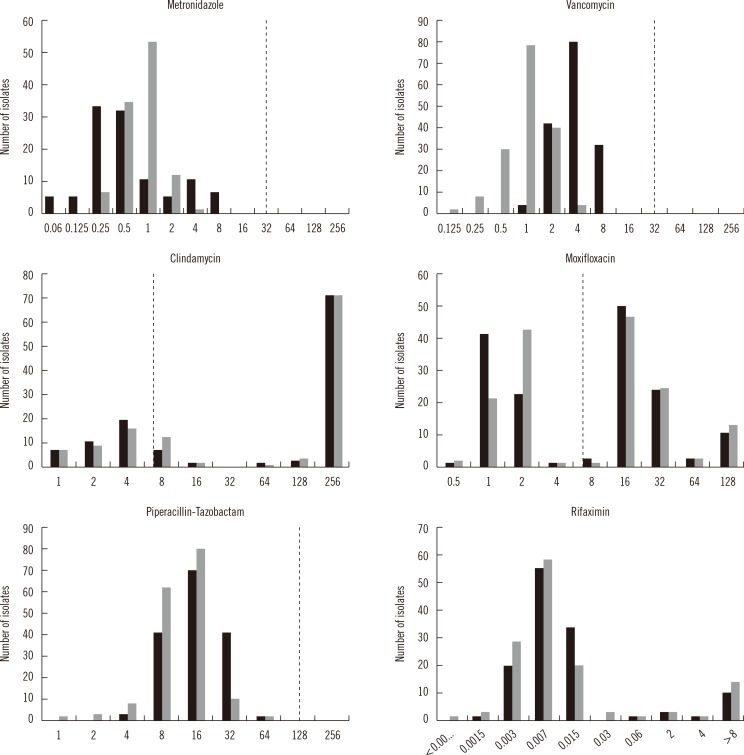
Table 1
The minimum inhibitory concentrations (MIC) range, MIC50, MIC90, and geometric mean MIC of the six antimicrobial agents against the 171 Clostridium difficile isolates determined by the agar dilution method on supplemented Brucella agar (sBA) and modified C. difficile (mCD) agar
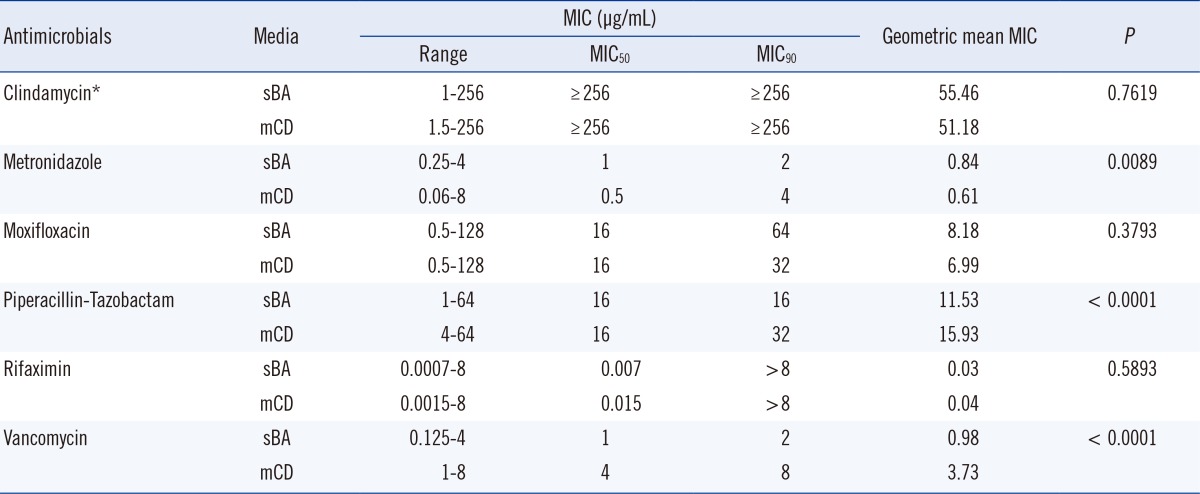
Table 2
Percent agreement for MIC results in double dilution differences for the six antimicrobial agents against 171 clinical isolates of Clostridium difficile on supplemented Brucella agar and modified C. difficile agar




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download