Anti-donor HLA-specific antibodies (DSA) are associated with poor graft outcomes in renal transplantations [1, 2]. Panel reactive antibody (PRA) assays using the Luminex platform are commonly used to detect DSA. PRA assays are performed by using three different panels: 1) pooled antigen panels that use bead populations coated in affinity-purified HLA molecules obtained from multiple cell lines, which are used as screening tests; 2) phenotype panels that use bead populations bearing HLA proteins from a cell line derived from a single individual; and 3) single-antigen bead (SAB) assays that use beads coated in molecules representing a single cloned allelic HLA antigen [3]. Each bead population in phenotype panels features more than one type of HLA molecule, thus requiring expertise to interpret the results, whereas SAB assays provide accurate identification of HLA antibodies [3]. However, cloned HLA antigens are not in their native state. Purification and bead coating can lead to conformational changes that could cause binding of clinically irrelevant antibodies [4, 5, 6, 7, 8, 9]. Here, we report a case with suspected false reactions against denatured HLA class II molecules in SAB assays.
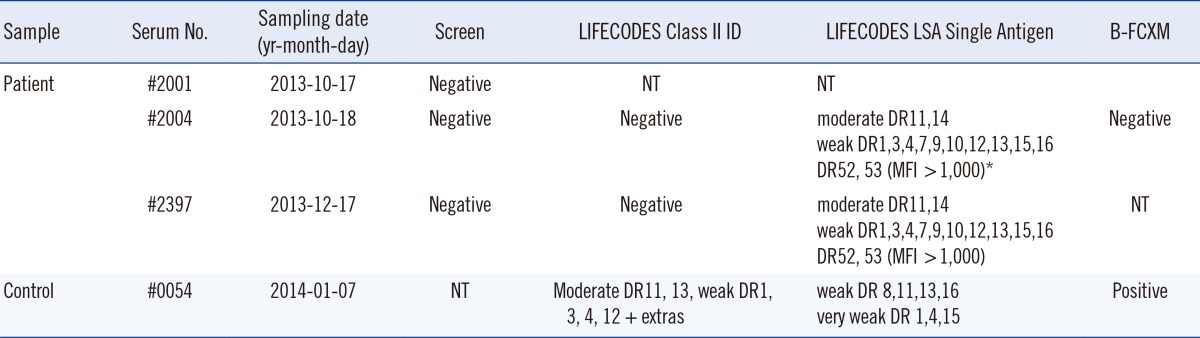
A 74-yr-old male patient who was monitored after penetrating keratoplasty and corneal allograft (November 7, 2012) was referred for PRA screening for anti-HLA antibodies. PRA screening was performed using the LIFECODES LifeScreen Deluxe kit (Immucor, Stamford, CT, USA). The serum drawn on October 17, 2014 (serum No. #2001) was negative for anti-HLA, but showed a slight increase in median fluorescence intensity (MFI) value in one of the four cross-reactive class II group beads. On the following day, a SAB assay was performed on newly collected serum (serum No. #2004) by using the LIFECODES LSA Class II Single Antigen kit (Immucor, lot. 10102A). The following positive reactions with HLA antibody specificities were obtained: 1) moderate DR11 and 14; 2) weak DR1, 3, 4, 7, 9, 10, 12, 13, 15, and 16; and 3) DR52 and 53 (MFI >1,000) (Table 1; moderate: MFI 3,000-9,999; weak: MFI 1,000-2,999). Two months later, a follow-up test performed using the LIFECODES Class II ID kit (Immucor) was found to be negative (serum No. #2397). A repeat test was performed on serum #2004 by using the LIFECODES LSA Class II Single Antigen kit to rule out clerical errors; the results obtained were the same as those obtained using the SAB assay performed previously. LIFECODES Screen and Class II ID tests were performed on serum #2004 and were negative. Finally, the LIFECODES Screen test performed on serum #2397 showed negative results, and a LIFECODES LSA Single Antigen test performed on serum #2397 by using a kit with a different lot number (06113A) showed results consistent with those of the previous SAB assay.
To rule out false-positive reactions in the SAB assays, flow cytometric crossmatches (FCXM) were performed on serum #2004 and serum from a second patient (#0054) with similar HLA antibody specificities as control: moderate anti-DR11 and 13; weak DR1, 3, 4, 12 + extras; and no HLA class I antibody in PRA ID tests. Lymphocytes from two donors with HLA-DR11, 13 and HLA-DR11, 14 were used for FCXM analysis. Serum #0054 showed positive B-cell (B)-FCXM to both donors, but serum #2004 was negative. In a previous study, we showed 90% of sera containing DSA with moderate MFI (3,000-9,999) in LIFECODES ID tests showed positive B-FCXM [10]. Because the LIFECODES LSA Single Antigen and LIFECODES ID MFI values were similar in our laboratory, the negative B-FCXM result on serum #2004 suggests moderate DSA is less likely. The patient showed a clear corneal allograft at the final follow-up on February 17, 2014.
So-called "natural" antibodies reacting with dissociated forms of HLA class I antigens have been reported in healthy male blood donors [8]. These antibodies could be anti-HLA-E, which is frequently found in healthy people [11] and usually shows low MFI values (less than 1,000) in SAB assays [8]. However, unpredictable false-positive reactions to denatured HLA antigens in SAB assays can show moderate to strong reactivity to both HLA class I [4, 5, 7] and class II antigens [6], which can lead to unnecessary exclusion of organ donors or immunosuppressive therapy. A patient with negative FCXM but positive DSA to denatured anti-A*01:01 in SAB assays, who received a kidney transplantation, had no rejection episodes in the 5 yr following transplantation [4]. Moreover, a patient with DSA to denatured HLA-A*02:01 showed no rejection one year after heart transplantation [5]. Among 8,121 consecutive samples tested using the LABScreen Single Antigen kit (One Lambda, Canoga Park, CA, USA), 141 sera samples (1.4%) showed an atypical reactivity to denatured HLA-DRB1*09:01, DRB3*01:01, *02:02, *03:01, DPB1*20:01, and *28:01 [6]. While most previous studies were performed using the LABScreen Single Antigen kit [4, 5, 6], one study reported identification of antibodies to denatured HLA-B*44:02 by using the LIFECODES LSA Single Antigen test [7]. To the best of our knowledge, we are the first to report identification of suspected antibodies to denatured HLA class II antigens by using the LIFECODES LSA Single Antigen kit, which is widely used in Korea. The limitation of our study is that we did not confirm that the reactivity was specifically toward denatured, but not intact antigens. Further, other possible reasons for false-positive SAB assay reactions could not be ruled out. However, we suggest that SAB assays should be used in conjunction with other types of PRA tests that use extracted HLA molecules to avoid unnecessary barriers to transplantation and inappropriate use of immunosuppressive therapy.
References
1. Mohan S, Palanisamy A, Tsapepas D, Tanriover B, Crew RJ, Dube G, et al. Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol. 2012; 23:2061–2071.

2. Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 2012; 8:348–357.

3. Zachary AA, Leffell MS. Detecting and monitoring human leukocyte antigen-specific antibodies. Hum Immunol. 2008; 69:591–604. PMID: 18692106.

4. Pereira S, Perkins S, Lee JH, Shumway W, LeFor W, Lopez-Cepero M, et al. Donor-specific antibody against denatured HLA-A1: clinically nonsignificant? Hum Immunol. 2011; 72:492–498.

5. Poli F, Benazzi E, Innocente A, Nocco A, Cagni N, Gianatti A, et al. Heart transplantation with donor-specific antibodies directed toward denatured HLA-A*02:01: a case report. Hum Immunol. 2011; 72:1045–1048.

6. Grenzi PC, de Marco R, Silva RZ, Campos EF, Gerbase-DeLima M. Antibodies against denatured HLA class II molecules detected in luminex-single antigen assay. Hum Immunol. 2013; 74:1300–1303.

7. Jacob EK, De Goey SR, Gandhi MJ. Positive virtual crossmatch with negative flow crossmatch results in two cases. Transpl Immunol. 2011; 25:77–81.

8. El-Awar N, Terasaki PI, Nguyen A, Sasaki N, Morales-Buenrostro LE, Saji H, et al. Epitopes of human leukocyte antigen class I antibodies found in sera of normal healthy males and cord blood. Hum Immunol. 2009; 70:844–853. PMID: 19580837.

9. Zoet YM, Brand-Schaaf SH, Roelen DL, Mulder A, Claas FH, Doxiadis II. Challenging the golden standard in defining donor-specific antibodies: does the solid phase assay meet the expectations? Tissue Antigens. 2011; 77:225–228.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download