Abstract
Background
Hypercalciuria is one of the most common causes of unexplained isolated hematuria. The diagnostic methods for hypercalciuria have not yet been standardized. The aim of this study was to assess whether random urinary calcium/creatinine ratio could be used as a screening tool for hypercalciuria in children with hematuria.
Methods
This prospective study included 264 children with primary hematuria for whom both random and 24 hr urinary evaluations were performed. Pearson correlation and ROC curve were used to assess the correlations. A multiple linear regression model was used to analyze effects of age, weight, height, body mass index, and body surface area on random urinary calcium/creatinine ratio.
Results
There was a moderately strong correlation between random urinary calcium/creatinine ratio and 24 hr urinary calcium excretion (r=0.584, P<0.001). The most appropriate cutoff value of random urinary calcium/creatinine ratio for the estimation of hypercalciuria was 0.075 mg/mg (sensitivity, 77.8%; specificity, 64.3%; area under the curve, 0.778). Body mass index and 24 hr urinary calcium excretion significantly affected random urinary calcium/creatinine ratio with a low coefficient of determination (r2=0.380, P<0.001).
Hypercalciuria is one of the most common causes of unexplained isolated hematuria and urinary stones in children [1]. Hypercalciuria can cause recurrent abdominal pain, flank pain, dysuria, urgency, nocturnal enuresis, and reduction in bone mineral density [2-4]. The prevalence of hypercalciuria varies in different countries and has been estimated at 0.6% in the Japanese children and 38.6% in the Kazakh children [5, 6]. In the Korean children, the prevalence of hypercalciuria was reported to be 4.8-6.3% [7, 8]. However, the methods used to diagnose hypercalciuria have not been standardized. Although the measurement of urinary calcium excretion using 24 hr urinary analysis is essential for the diagnosis of hypercalciuria, the procedure is often difficult to apply in young children. Thus, random urinary calcium/creatinine ratio (UCa/Cr) has been used to overcome such difficulties. However, the replacement of 24 hr urinary calcium excretion (24hCa) with UCa/Cr is controversial. Some reports have found strong correlations [9, 10], but the other studies have not found convincing evidence [11, 12]. Furthermore, the cutoff value of UCa/Cr that would allow screening for hypercalciuria has not been established precisely, and this ratio varies with age and geographic location [13-15]. In Korea, UCa/Cr ≥0.20 mg/mg has been used as an accepted cutoff value for screening children of 3 yr or more with hypercalciuria [16].
Most previous studies on the correlation between UCa/Cr and 24hCa have involved healthy children [9, 11, 17]. In the literature, few studies have published data on the correlation of UCa/Cr and 24hCa with symptoms of potential hypercalciuria [10, 12]. The aims of this study were to determine the correlation between UCa/Cr and 24hCa and to assess whether UCa/Cr could be used for screening hypercalciuria in children with isolated gross or microscopic hematuria.
This prospective study was conducted in the pediatric nephrology clinic of our hospital between January 2006 and June 2011. Patients who presented with isolated gross or microscopic hematuria were enrolled in the study. Gross hematuria was defined as visible bloody urine that occurred one or more times. Microscopic hematuria was detected by the primary care physician in a random urine specimen and was confirmed by a microscopic examination of the urine sediment showing more than 5 red blood cells per high-power field. Exclusion criteria were as follows: 1) not toilet-trained, 2) receiving any drugs, 3) decreased renal function, 4) diagnosed with any glomerular disease, 5) diagnosed with nephrolithiasis, or 6) diagnosed with infectious disease at the time of presentation. Physical examinations were performed for all enrolled patients, and their heights and weights were noted.
The study protocol was approved by the Institutional Review Board of our hospital. The recommendations of the Declaration of Helsinki for biomedical research involving human subjects were followed.
Patients were given a urine collection cup, a urine container, and written instructions for urine collection. Each subject was asked to collect a 24 hr urine sample the day before arrival at the hospital. The first morning urine was discarded on the first day, and each void of that day was continuously collected along with the first morning urine of the next day. Fasting random urine volume of 10 mL was collected from the last voiding urine; therefore, the volume collected was the same as that obtained at the first morning urine; this was considered the end of the 24 hr collection.
Urinary calcium concentration was measured by an automated chemistry analyzer using the o-cresolphthalein complexone method with the Calcium C-test (Wako Pure Chemical Industries, Osaka, Japan). The solution was diluted with an appropriate volume of distilled water. Urinary creatinine was analyzed by the same chemical analyzer using the standard Jaffe kinetic reaction with picric acid (Roche Diagnostic Systems, Inc., Montclair, NJ, USA).
UCa/Cr (mg/mg) was calculated as random urinary calcium (mg/dL) excretion divided by random urinary creatinine excretion (mg/dL). 24hCa (mg/kg/day) was calculated by as the 24 hr urinary calcium excretion divided by body weight. Body surface area (BSA, m2) was calculated according to the Mosteller formula [18]. Body mass index (BMI, kg/m2) was calculated as the ratio between weight and the square of height. Children with a daily urinary calcium excretion of more than 4 mg/kg/day were considered to have definite hypercalciuria [9].
Pearson correlation analysis and the ROC curve were used to assess the correlation between random and 24 hr urinary samples. An ROC curve was constructed to establish the cutoff value of UCa/Cr that would be indicative of a high probability of hypercalciuria in the 24 hr urine. A multiple linear regression model was used to investigate correlations among factors, including age, body height, body weight, BMI, BSA, 24hCa, and UCa/Cr. Statistical significance was set at P<0.05. All statistical analyses were performed with SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA).
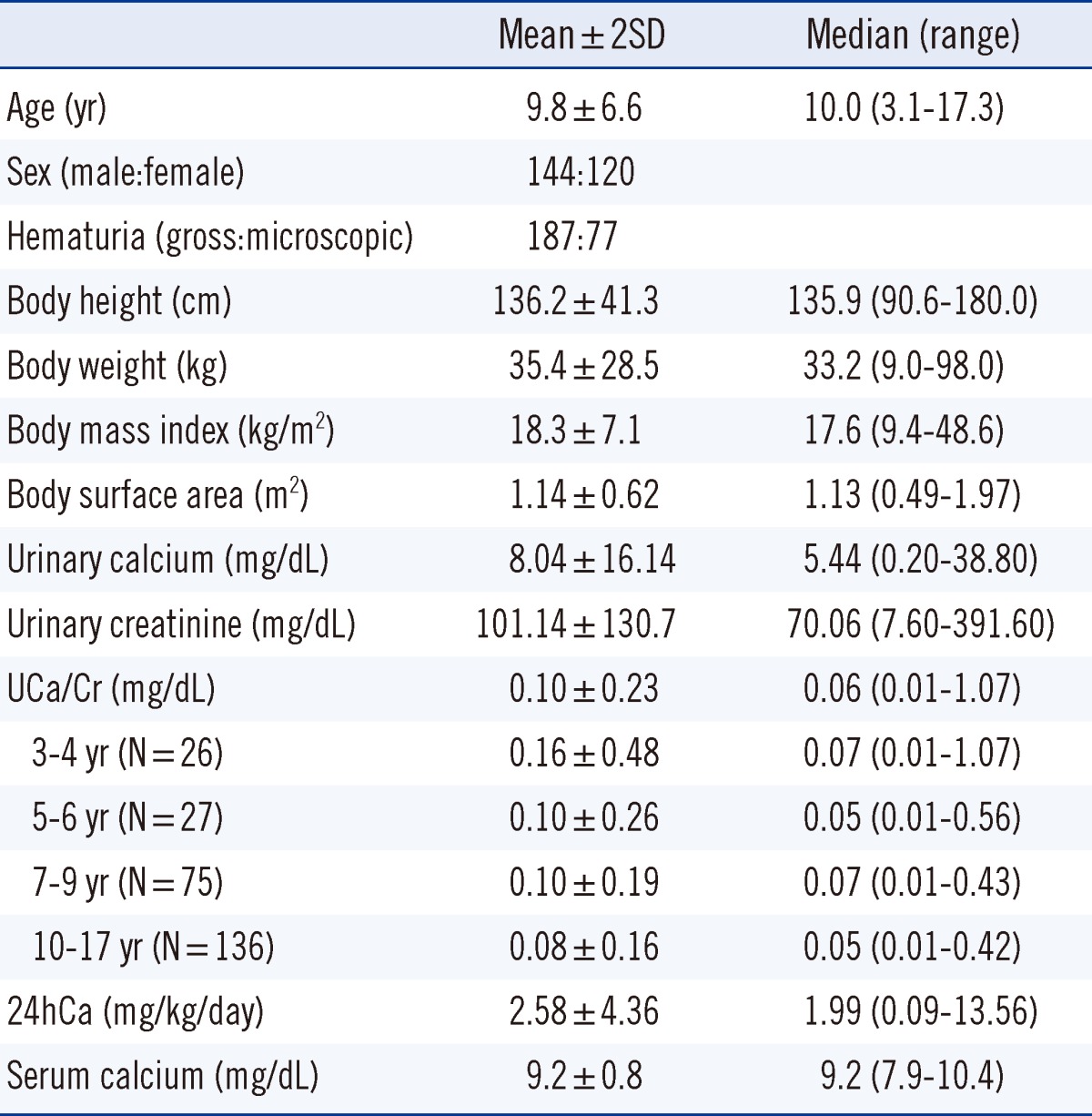
A total of 264 patients were enrolled in this study. In all, 187 patients (70.8%) had microscopic hematuria and 77 patients (29.2%) had gross hematuria. The patients' age, body size, and results of the urinary evaluations are shown in Table 1. Age ranged from 3 to 17 yr, body height from 90.6 to 180.0 cm, body weight from 9 to 98 kg, and BSA from 0.49 to 1.97 m2. The mean 24hCa was 2.58±2.18 mg/kg/day. The mean UCa/Cr was 0.10±0.09 mg/mg, and the mean UCa/Cr values according to each age group are shown in Table 1.
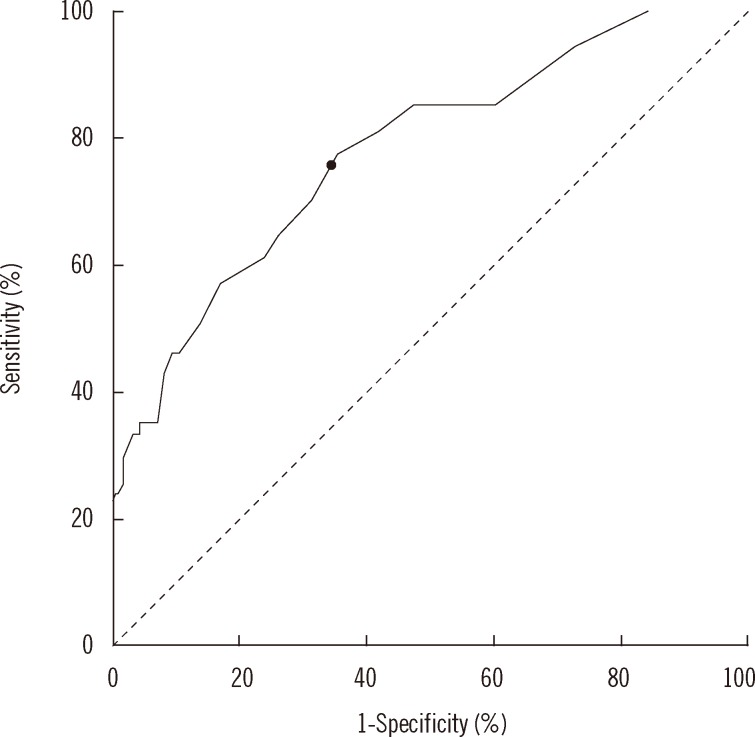
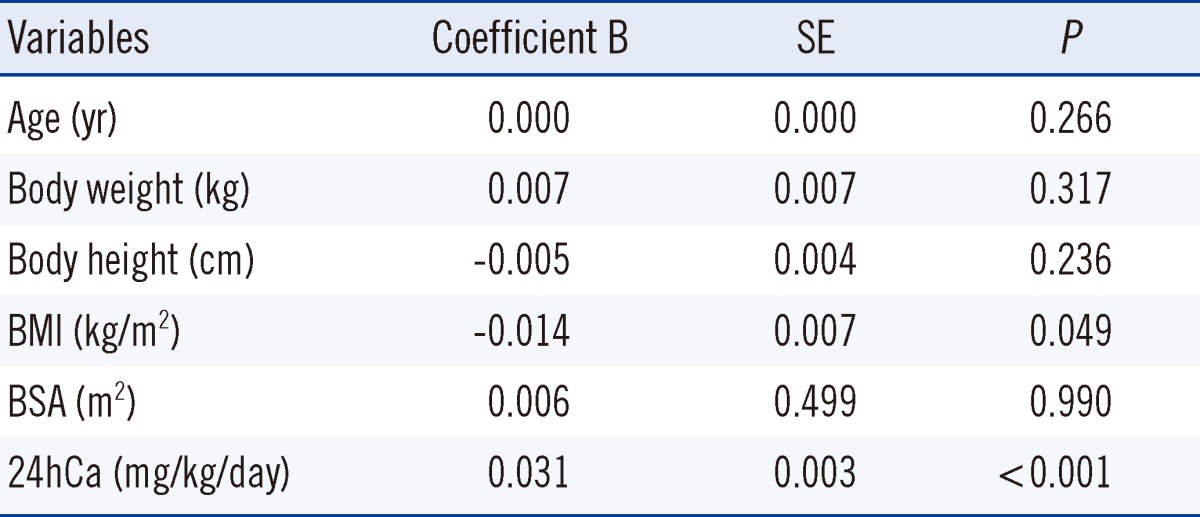
Pearson correlation analysis was performed to assess the correlation between UCa/Cr and 24hCa. We found a positive linear correlation that was statistically significant (P<0.001). The Pearson coefficient (r) for UCa/Cr and 24hCa was 0.584, showing a moderate correlation (Fig. 1). In addition, the Pearson coefficient (r) in 187 patients with microscopic hematuria was 0.597 (P<0.001) and 0.596 in 77 patients with gross hematuria (P<0.001). There were moderate correlations between UCa/Cr and 24hCa in both groups. In the ROC curve, the most appropriate cutoff value of UCa/Cr for estimating hypercalciuria was 0.075 mg/mg (sensitivity, 77.8%; specificity, 64.3%; area under the curve, 0.778; P<0.001; Fig. 2). The positive and negative predictive values were 77.7% and 64.3%, respectively, for UCa/Cr of 0.075 mg/mg. The commonly used UCa/Cr value of ≥0.20 mg/mg for screening for hypercalciuria showed low sensitivity (sensitivity, 35.2%; specificity, 93.3%) on the ROC curve. The multiple linear regression analysis with a dependent variable of UCa/Cr and independent variables of 24hCa, age, body weight, height, BMI, and BSA revealed that BMI, in addition to 24hCa, affected UCa/Cr with a low coefficient of determination (r2=0.380, P<0.001, Table 2).
Hematuria in children is one of the most common symptoms caused by hypercalciuria [1]. Clinicians should consider the possibility of hypercalciuria in children with hematuria. Urinary calcium excretion is best measured with a 24 hr collection. Because such collection methods are difficult to obtain in children, UCa/Cr has been often used as an alternative measurement. However, the correlation between UCa/Cr and 24hCa is controversial. Reusz et al. [19] reported a close linear correlation between calcium/creatinine ratio in the first-morning urine sample and calcium/creatinine ratio determined from 24 hr urine specimens in 94 children. Alconcher et al. [20], however, found a weak correlation between fasting UCa/Cr ratio and 24hCa in 220 school-aged children. Koyun et al. [11] reported a weak correlation between non-fasting, second morning UCa/Cr ratio and daily urinary calcium excretion in a larger sample of 269 children. In the present study, UCa/Cr was not appropriate for screening hypercalciuria in children with hematuria. In addition, the commonly used cutoff value of UCa/Cr ≥0.20 mg/mg could not predict the presence of hypercalciuria in an ROC analysis (sensitivity, 35.2 %; specificity, 93.3 %).
Several studies have reported significant age-related variations in UCa/Cr [13, 14, 21, 22]. Esbjörner and Jones [21] found a weak but significant negative correlation between postprandial UCa/Cr in a group of children aged 2-18 yr (N=153). Sargent et al. [22] found an age-related decrease in UCa/Cr in children<6 yr of age but did not specify the age at which UCa/Cr values became stable. Safarinejad [14] reported that the 95th percentile for UCa/Cr decreased progressively after 7 yr of age. Since creatinine is derived from creatine in muscle, its urinary excretion is dependent on the muscle mass of the subject [23]. Mori et al. [24] reported that urinary creatinine excretion depended on the sex and BSA in children. In the present study, UCa/Cr showed a decreasing trend with increasing age, although this finding was not significant (Table 1). BMI was significantly correlated with UCa/Cr in the multiple linear regression model (Table 2). However, the regression equation, which included BMI, had a low coefficient of determination (r2=0.380).
Urinary calcium excretion varies with age and is the highest in young children and reaching its nadir during puberty [25]. There are conflicting data in the literature concerning the correlation between urinary calcium excretion and sex. Seifert-McLean et al. [26] found sex to be a significant factor for calcium excretion, but other researchers have shown conflicting results [13, 27, 28]. Slev et al. [28] reported that sex difference was not significant for calcium excretion, but boys had lower concentrations than girls in the corresponding age group. Individual variations in dietary calcium intake may also be a source of the variation in urinary calcium excretion. Additional factors, including geography, race, water source, exposure to sunlight, and even season, have been proposed to influence urinary calcium concentration.
Recent studies on the correlation between UCa/Cr and 24hCa have been concerned with healthy children. The hypothesis of this study was that UCa/Cr might be more closely correlated with 24hCa in patients with potential hypercalciuria. In a recent report that enrolled 50 adults with calcium lithiasis, researchers found a strong and positive linear correlation between the random fasting and 24 hr urine for calcium/creatinine ratio according to Pearson correlation analysis (r=0.725, P=0.0001). In addition, the cutoff value of calcium level in the random fasting urine for screening hypercalciuria showed high sensitivity in a ROC curve (cut-off value, 10.15 mg/dL; sensitivity, 89%; specificity, 59%; area under curve, 0.765; P=0.003) [10]. When targeted at adults, random urine samples might be useful for screening hypercalciuria in patients with calcium lithiasis. However, in the present study, UCa/Cr showed relatively low sensitivity and specificity for screening hypercalciuria in children with hematuria.
The present study has several limitations. The major limitation is that we did not have a group of normal children for comparison. However, when our data were compared with previous studies including normal children, there were no great discrepancies in the area under curve or coefficient of determination. Another limitation is that other symptoms, such as a dysuria and abdominal or flank pain associated with potential hypercalciuria, were not included in the study. To our knowledge, no reported studies have evaluated the correlation between UCa/Cr and 24hCa targeted to children with hematuria.
In conclusion, there is a moderate correlation between UCa/Cr and 24hCa in children with hematuria. UCa/Cr is not suitable for screening hypercalciuria in children with hematuria. Twenty-four hours urinary analysis is crucial for the diagnosis of hypercalciuria in children with hematuria.
References
1. Feld LG, Meyers KE, Kaplan BS, Stapleton FB. Limited evaluation of microscopic hematuria in pediatrics. Pediatrics. 1998; 102:E42. PMID: 9755279.

2. Vachvanichsanong P, Malagon M, Moore ES. Recurrent abdominal and flank pain in children with idiopathic hypercalciuria. Acta Paediatr. 2001; 90:643–648. PMID: 11440097.

3. Zerwekh JE. Bone disease and hypercalciuria in children. Pediatr Nephrol. 2010; 25:395–401. PMID: 19885683.

4. Pace G, Aceto G, Cormio L, Traficante A, Tempesta A, Lospalluti ML, et al. Nocturnal enuresis can be caused by absorptive hypercalciuria. Scand J Urol Nephrol. 1999; 33:111–114. PMID: 10360451.
5. Kaneko K, Tsuchiya K, Kawamura R, Ohtomo Y, Shimizu T, Yamashiro Y, et al. Low prevalence of hypercalciuria in Japanese children. Nephron. 2002; 91:439–443. PMID: 12119474.

6. Kaneko K, Chiba M, Hashizume M, Kunii O, Sasaki S, Shimoda T, et al. Extremely high prevalence of hypercalciuria in children living in the Aral Sea region. Acta Paediatr. 2002; 91:1116–1120. PMID: 12434899.

7. Ryu KH, Lee SJ, Lee K, Jung JS. Idiopathic hypercalciuria in children. J Korean Pediatr Soc. 1989; 32:809–815.
8. Ahn YH, Kim KH, Ko CW, Koo JH. Idiopathic hypercalciuria in children. Korean J Nephrol. 1989; 8:79–84.
9. Ghazali S, Barratt TM. Urinary excretion of calcium and magnesium in children. Arch Dis Child. 1974; 49:97–101. PMID: 4406087.

10. Arrabal-Polo MA, Arias-Santiago S, Girón-Prieto MS, Abad-Menor F, López-Carmona Pintado F, Zuluaga-Gomez A, et al. Hypercalciuria, hyperoxaluria, and hypocitraturia screening from random urine samples in patients with calcium lithiasis. Urol Res. 2012; 40:511–515. PMID: 22484727.

11. Koyun M, Güven AG, Filiz S, Akman S, Akbas H, Baysal YE, et al. Screening for hypercalciuria in schoolchildren: what should be the criteria for diagnosis? Pediatr Nephrol. 2007; 22:1297–1301. PMID: 17549524.

12. Hong YH, Dublin N, Razack AH, Mohd MA, Husain R. Twenty-four hour and spot urine metabolic evaluations: correlations versus agreements. Urology. 2010; 75:1294–1298. PMID: 19914693.

13. Matos V, van Melle G, Boulat O, Markert M, Bachmann C, Guignard JP. Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr. 1997; 131:252–257. PMID: 9290612.

14. Safarinejad MR. Urinary mineral excretion in healthy Iranian children. Pediatr Nephrol. 2003; 18:140–144. PMID: 12579403.

15. Sönmez F, Akcanal B, Altincik A, Yenisey C. Urinary calcium excretion in healthy Turkish children. Int Urol Nephrol. 2007; 39:917–922. PMID: 17043921.

16. Ahn HS, Suh BK, Seo JK, Oh SH, Lee SI, Lee C, editors. Textbook of Pediatrics. 10th ed. Seoul: Miraen Publishing Co.;2012. p. 916–917.
17. Mir S, Serdaroqlu E. Quantification of hypercalciuria with the urine calcium osmolality ratio in children. Pediatr Nephrol. 2005; 20:1562–1565. PMID: 16133062.

18. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987; 317:1098. PMID: 3657876.

19. Reusz GS, Dobos M, Byrd D, Sallay P, Miltenyi M, Tulassay T. Urinary calcium and oxalate excretion in children. Pediatr Nephrol. 1995; 9:39–44. PMID: 7742220.

20. Alconcher LF, Castro C, Quintana D, Abt N, Moran L, Gonzalez L, et al. Urinary calcium excretion in healthy school children. Pediatr Nephrol. 1997; 11:186–188. PMID: 9090660.

21. Esbjörner E, Jones IL. Urinary calcium excretion in Swedish children. Acta Paediatr. 1995; 84:156–159. PMID: 7756801.
22. Sargent JD, Stukel TA, Kresel J, Klein RZ. Normal values for random urinary calcium to creatinine ratios in infancy. J Pediatr. 1993; 123:393–397. PMID: 8355114.

23. Remer T, Neubert A, Maser-Gluth C. Anthropometry-based reference values for 24-hr urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr. 2002; 75:561–569. PMID: 11864864.
24. Mori Y, Hiraoka M, Suganuma N, Tsukahara H, Yoshida H, Mayumi M. Urinary creatinine excretion and protein/creatinine ratios vary by body size and gender in children. Pediatr Nephrol. 2006; 21:683–687. PMID: 16550362.

25. Manz F, Kehrt R, Lausen B, Merkel A. Urinary calcium excretion in healthy children and adolescents. Pediatr Nephrol. 1999; 13:894–899. PMID: 10603144.

26. Seifert-McLean CM, Cromer BA, Mosher G, Mahan JD. Urinary calcium excretion in healthy adolescents. J Adolesc Health Care. 1989; 10:300–304. PMID: 2732110.

27. Metz MP. Determining urinary calcium/creatinine cut-offs for the paediatric population using published data. Ann Clin Biochem. 2006; 43:398–401. PMID: 17022882.

28. Slev PR, Bunker AM, Owen WE, Roberts WL. Pediatric reference intervals for random urine calcium, phosphorus and total protein. Pediatr Nephrol. 2010; 25:1707–1710. PMID: 20473690.

Fig. 1
Pearson correlation between random urinary calcium/creatinine ratio and 24 hr urinary calcium excretion. The Pearson coefficient (r) was 0.584 (P<0.001).
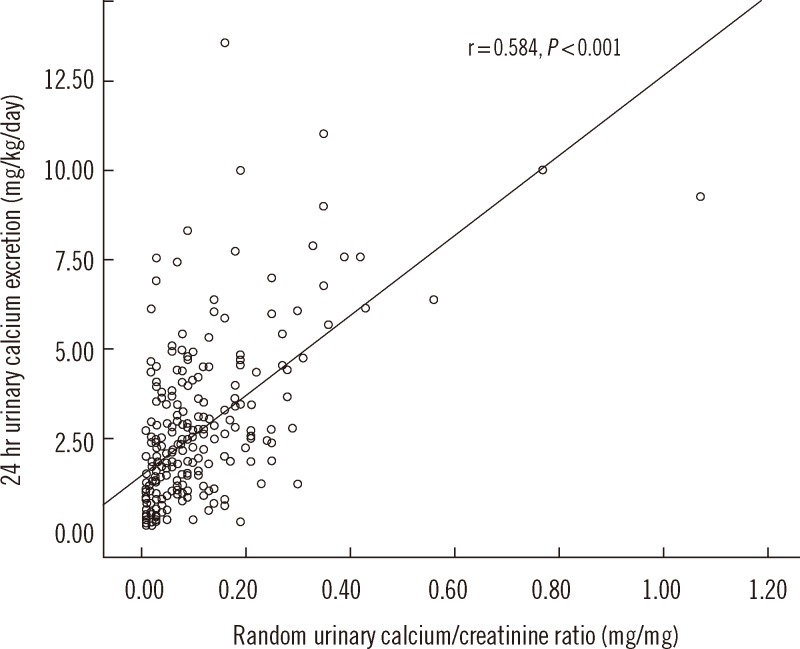
Fig. 2
Receiver operating characteristics curve analysis of random urinary calcium/creatinine ratio. The cut-off value (marked with a black dot on the curve) of random urinary calcium/creatinine ratio for detecting hypercalciuria was defined as 0.075 mg/mg (sensitivity, 77.8%; specificity, 64.3%; area under the curve, 0.778; P<0.001).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download