Dear Editor,
Burkitt lymphoma/leukemia (BL/L) was the first neoplasia associated with rearrangement in MYC that is the molecular hallmark of this disease. Particularly, immunoglobulin genes are associated with MYC in BL/L, where the juxtaposition with heavy chain locus (14q32) or light chain loci (2p12 and 22q11) leads to MYC overexpression, which is believed to play a central role in BL/L pathogenesis [1].
During investigation of the clinical significance of secondary chromosomal abnormalities in pediatric BL/L, we identified a patient with BL/L without detectable MYC translocation [2]. Here, we aimed to refine cytogenetic and molecular characteristics, such as expression levels of MYC, other genes, and microRNAs, to contribute to the diagnosis of BL/L without MYC translocations. Clinical data are described in Table 1.
G-banding of bone marrow (BM) cells revealed 46,XY,der(8) in 31.5% of the metaphases analyzed (Fig. 1A). FISH analysis revealed three MYC signals, two in the derivative chromosome 8, apart from a normal chromosome 8 (Fig. 1B, C). FISH analysis of BCL6 and BCL2 loci ruled out abnormalities common to other B cell non-Hodgkin lymphomas (NHL) (Fig. 1D). Multicolor FISH excluded the presence of any other chromosomal abnormality, including changes in chromosome 11, recently associated with MYC-negative BL/L cases in the WHO classification [3]. Multicolor probes helped define the final karyotype as 46,XY,der(8)t(8;8)(qter->q21::p22->qter) (Fig. 1E, F).
In 2006, Hummel et al [4] proposed a molecular signature for BL/L, including in their sample cases with lymphomas lacking MYC rearrangement. Among them, CD10 and BCL2, besides MYC, were found to be differentially expressed and are used as classifiers for BL/L signature. Moreover, supporting the idea of MYC post-transcriptional deregulation in BL/L, some studies have revealed differential expression patterns of specific miRNAs in comparison to that in other NHL [56]. For molecular characterization, tumor samples from four patients with BL/L harboring t(8;14)(q24;q32) (median age, nine years; BCL6-positive and BCL2-negative), cells from three BL and two diffuse large B-cell lymphoma cell lines, three reactive follicular hyperplasia lymph nodes, and two normal BM samples were used for comparison.
MYC and BCL2 expression levels in our patient were similar to those in the BL/L group, although BCL2 levels were lower than those observed in all the cases with BL/L (Fig. 1G, H). Similarly, CD10 levels were lower than those in the BL/L group (Fig. 1I). These differences are likely to have arisen from the different types of samples used for molecular testing. miR155 and Let7a, 7b, and 7e, which are downregulated by MYC [7], were in general at low levels in the BL/L group (Fig. 1J–M). miR9*, usually downregulated in patients lacking the MYC translocation [6], was downregulated in our patient as well as in the BL/L group (Fig. 1N). miR150 and miR21 were downregulated in all the cases (Fig. 1O–P) [7]. Thus, gene expression analysis of our patient suggests a BL-like molecular profile despite the lack of MYC translocation.
To the best of our knowledge, this is the first report on a patient with a partial trisomy 8 lacking the typical t(8;14)(q24;q32), which resulted in three copies of MYC, and MYC overexpression was comparable to that generally found in BL/L. In rare cases, MYC rearrangement cannot be identified [15], and the gene expression profile appears to be comparable to that observed in BL/L [4]. This suggests that other pathogenic mechanisms could lead to deregulation, such as post-transcriptional control by microRNA, of MYC expression [56]. In this context, since 2008, the WHO classification includes BL/L cases without a demonstrable MYC translocation [3].
Ataxia-telangiectasia (A-T) is a rare neurodegenerative disorder associated with an elevated risk (10–30%) of developing malignancies. NHL was the most frequently detected cancer (53–64%) in patients with A-T [8]; however, BL/L is rarely reported. Despite its rarity, Sandlund et al [9] suggested that BL/L in patients with A-T tends to carry non-canonical MYC rearrangements, probably because of global chromosome instability. This hypothesis is in agreement with that observed in our patient.
In summary, our results, obtained using molecular cytogenetics and expression approaches, add new information about BL/L without MYC translocation. Whether the altered MYC expression in our patient resulted from microRNA deregulation, a known alternative pathogenic mechanism [56], or from trisomy 8, which might result in MYC overexpression by an increased gene dosage, remains to be elucidated.
Acknowledgments
CAPES-PROBRAL/DAAD (419/14), Ministèrio da Saúde, Monika Kutzner Stiftung (Germany), the St. Jude Children's Research Hospital, and the Center of Excellence Grant from the State of Tennessee (USA).
References
1. Greenought A, Dave SS. New clues to the molecular pathogenesis of Burkitt lymphoma revealed through next-generation sequencing. Curr Opin Hematol. 2014; 21:326–332. PMID: 24867287.
2. De Souza MT, Hassan R, Liehr T, Marques-Salles TJ, Boulhosa AM, Abdelhay E, et al. Conventional and molecular cytogenetic characterization of Burkitt lymphoma with bone marrow involvement in Brazilian children and adolescents. Pediatr Blood Cancer. 2014; 61:1422–1469. PMID: 24668946.
3. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127:2375–2390. PMID: 26980727.
4. Hummel M, Bentink S, Berger H, Klapper W, Wessendorf S, Barth TFE, et al. Molecular mechanisms in Malignant Lymphomas Network Project of the Deutsche Krebshilfe. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006; 354:2419–2430. PMID: 16760442.
5. Onnis A, De Falco G, Antonicelli G, Onorati M, Bellan C, Sherman O, et al. Alteration of microRNAs regulated by c-Myc in Burkitt lymphoma. PloS One. 2010; 5:pii: e12960.
6. Leucci E, Cocco M, Onnis A, De Falco G, van Cleef P, Bellan C, et al. MYC translocation-negative classical Burkitt lymphoma cases: an alternative pathogenic mechanism involving miRNA deregulation. J Pathol. 2008; 216:440–450. PMID: 18802929.
7. Robertus JL, Kluiver J, Weggemans C, Harms G, Reijmers RM, Swart Y, et al. miRNA profiling in B non-Hodgkin lymphoma: a MYC-related miRNA profile characterizes Burkitt lymphoma. Br J Haematol. 2010; 149:896–899. PMID: 20331457.
8. Schoenaker MHD, Suarez F, Szczepanski T, Mahlaoui N, Loeffen JL. Treatment of acute leukemia in children with ataxia-telangiectasia (A-T). Eur J Med Genet. 2016; 59:641–646. PMID: 27238889.
9. Sandlund JT, Hudson MM, Kennedy W, Onciu M, Kastan MB. Pilot study of modified LMB-based therapy for children with ataxia-telangiectasia and advanced stage high grade mature b-cell malignancies. Pediatr Blood Cancer. 2014; 61:360–362. PMID: 23900766.
10. Vera-Lozada G, Scholl V, Barros MH, Sistic D, Guescinic M, Stocch V, et al. Analysis of biological and technical variability in gene expression assays from formalin-fixed paraffin-embedded classical Hodgkin lymphomas. Exp Mol Pathol. 2014; 97:433–439. PMID: 25236575.
Fig. 1
Cytogenetics and molecular characterization. (A) G-banding cytogenetics: GTG banding karyotype showing the derivative chromosome 8 pointed by the red arrow. (B–F) Molecular cytogenetics: (B) FISH analysis using IGH/MYC/CEP8 Tri-Color Dual Fusion Probe (04N10-020, Abbott Molecular, Des Plaines, IL, USA). Green signal: IGH; red signal: MYC; aqua signal: CEP8; (C) LSI MYC Spectrum Orange Probe (02N22-020, Abbott Molecular) shows derivative chromosome 8 with 2 copies of MYC, 3 in total per cell; (D) Complementary FISH analyses using BCL6 Break Apart Probe (Z-2177-50, ZytoVision GmbH, Bremerhaven, HB, DE) and BLC2 Break Apart Probe (Z-2192-50, ZytoVision GmbH) showed normal partners for both chromosomes 3 and 18, respectively; (E) FISH using partial chromosome paintings for 8p and 8q arms showed partial trisomy 8; (F) Multicolor chromosome banding probe for chromosome 8 characterized the derivative chromosome 8 as a result of t(8;8)(pter->q21::p22->qter); (G–P) Comparisons between cellular genes and microRNA (miRNA) expressions among our patient and classical Burkitt lymphomas, healthy bone marrow (BM) cells, reactive follicular hyperplasias (RFH), and BL- and diffuse large B-cell lymphoma (DLBCL)-derived cell lines. (G) MYC; (H) BCL2; (I) CD10; (J) miR-155; (K) miR-Let7a; (L) miR-Let7b; (M) miR-Let7e; (N) miR-9*; (O) miR-150; (P) miR-21. Case: study patient; BL: classical Burkitt lymphoma; BL-CL: represent the mean values of BL-derived cell lines—Namalwa, Raji, and Ramos; DLBCL-CL: represents the mean values of diffuse large B-cell lymphoma (DLBCL)-derived cell lines—Farage and Pfeiffer; BM: bone marrow cells from healthy donors; RFH: reactive follicular hyperplasia of lymph nodes. The line set at 1 represents the calibration reference. RNA was extracted from FFPE BM biopsy (case) and BL and RFH lymph node using MasterPure™ RNA Purification Kit (Epicentre, Madison, WI, USA). RNA from BM and cell lines was extracted with Direct-zol™ RNA MiniPrep (Zymo Research, Irvine, CA, USA). Relative expression of CD10 and BCL2 was evaluated by TaqMan® assays, as previously described [10], using the average of ACTB and B2M reference genes for normalization. MYC expression was quantified with SYBR green® assays using the average of ACTB and GUSB for normalization. miRNAs were quantified with stem-loop TaqMan® assays (Applied Biosystems, Life Technologies, Carlsbad, CA, USA) after reverse transcription with MicroRNA Reverse Transcription Kit (Applied Biosystems, Life Technologies) for each miRNA and the reference small RNA RNU48. Quantification values were expressed as fold change (2−ΔΔCq) after calibration with the classical BL sample exhibiting the lowest expression level. Bars represent the mean of fold change values in each category, except for the case, in which the mean of two different experiments was represented. Error bars represent standard error of the mean.

Table 1
Clinical characteristics of the patient
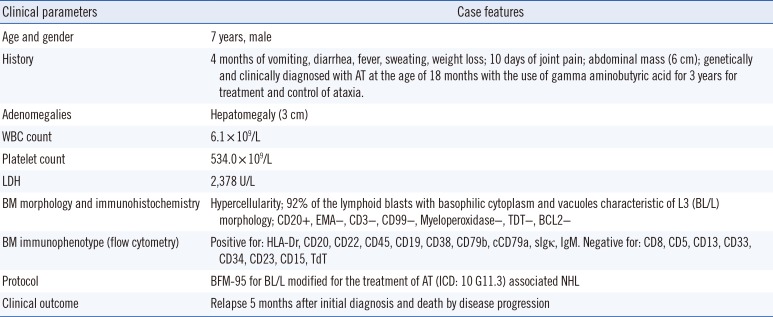
Abbreviations: cm, centimeters; AT, ataxia-telangiectasia; L, liter; U/L, units per liter; WBC, white blood cell; LDH, lactate dehydrogenase; BM, bone marrow; BL/L, Burkitt lymphoma/leukemia; BFM-95, Berlin, Frankfurt and Munster 95 protocol; ICD, International Classification of Diseases; NHL, non-Hodgkin lymphoma.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download