Abstract
Purpose
To investigate prognostic factors related to the surgical outcome of vitrectomy in myopic traction maculopathy (MTM).
Methods
Medical records of patients with MTM who underwent pars plana vitrectomy with internal limiting membrane peeling and follow-up over 12 months were reviewed retrospectively. Best-corrected visual acuity (BCVA), fundoscopic examination and spectral-domain optical coherence tomography findings were evaluated postoperatively. Functional success was defined as visual acuity gain and anatomical success was defined as reduction or resolution of foveoschisis without complications.
Results
This study included 40 eyes of 36 patients. BCVA improved from 0.70 ± 0.44 to 0.63 ± 0.57 logarithm of minimum angle of resolution and central macular thickness decreased from 526.6 ± 132.1 to 277.8 ± 92.1 µm at final follow-up. Functional success was achieved in 24 (60.0%) eyes, and 33 (82.5%) eyes reached anatomical success. Presence of foveal detachment (FD) and higher category of myopic maculopathy were associated with both functional (p = 0.014, 0.021, respectively) and anatomical (p = 0.011, 0.022, respectively) failure. Longer preoperative axial length showed an association with functional failure but not with anatomical failure (p = 0.041). In multivariate analysis, FD was the only prognostic factor for both functional and anatomical outcome (p = 0.041, 0.043, respectively). Preoperative BCVA (r2 = 0.259, p = 0.001), axial length (r2 = 0.172, p = 0.008), and myopic maculopathy category (r2 = 0.336, p < 0.001) showed significant correlation with final BCVA.
Pathologic myopia is a major cause of visual impairment especially in the Asian population [1]. In highly myopic eyes, posterior elongation of the sclera and the development of staphyloma result in retinal deformations, which lead to the development of myopic traction maculopathy (MTM). MTM is a critical complication that can threaten vision and is reported to occur in 9% to 34% of highly myopic eyes [23]. MTM includes structural features such as vitreomacular traction (VMT), macular retinoschisis, lamellar hole, or foveal detachment (FD). VMT, remnant cortical vitreous layer, epiretinal membrane (ERM), intrinsic noncompliance of inner limiting membrane, and inflexibility of retinal vessels could contribute to the pathogenesis of MTM [4567].
A study of the natural course of MTM indicated that more extensive macular retinoschisis had a higher probability of progression, and retinoschisis was resolved after the surgical release of VMT [8]. Pars plana vitrectomy (PPV) with internal limiting membrane (ILM) peeling has been reported to be effective for the resolution of macular retinoschisis [791011]. However, visual improvement was not significant in some patients and postoperative complications, such as macular hole (MH), have also been reported [1213].
Preoperative factors predicting surgical outcome in MTM are little known. In previous cases, preoperative visual acuity and axial length have been reported to be correlated with visual outcome [141516] and photoreceptor layer defects or irregular choroidal surfaces were reported to limit visual recovery [1718]. However, whether FD is a poor prognostic factor for surgical success is controversial [1419]. For these reasons, more studies are required to elucidate the predictive factors of vitrectomy in MTM.
In this study, we investigated prognostic factors related with the functional and anatomical outcomes of vitrectomy in MTM after more than one year of postoperative follow-up.
This study was approved by the institutional review board of the Seoul National University Bundang Hospital (B-1904/534-102) and the study was conducted according to the tenets of the Declaration of Helsinki. Written informed consent was waived due to the retrospective nature of the study. The medical records of 56 eyes of 50 patients with MTM who underwent PPV from March 2007 to December 2017, were retrospectively reviewed. Patients were excluded if they had a previous history of intraocular surgery other than cataract extraction or coexistent retinal diseases, follow-up less than 12 months, axial length shorter than 26 mm, MH or myopic choroidal neovascularization. Consequently, 40 eyes of 36 patients who met the inclusion criteria were included in the study. Functional success was defined as an improvement of visual acuity at the final visit compared with initial best-corrected visual acuity (BCVA). Anatomical success was defined as a reduction or resolution of schisis without complications, such as retinal detachment, MH formation, or recurrence.
PPV was performed by 2 experienced retinal specialists (SJW and KHP). A 23/25-gauge transconjunctival sutureless vitrectomy was performed using an Accurus 800CS surgical system (Alcon, Fort Worth, TX, USA) or Constellation system (Alcon) with contact lens (Hoya, Tokyo, Japan). Phacoemulsification with intraocular lens implantation was performed before vitrectomy in patients with significant cataract. The peeling of the ILM was performed using end-gripping forceps (Alcon). Triamcinolone acetonide (1%; Hanmi Pharmaceutical, Seoul, Korea) or 0.05% indocyanine green (Dong In Dang Pharmaceutical, Siheung, Korea) were used for staining and peeling of ILM. Foveal sparing ILM peeling was performed in 7 eyes and complete ILM peeling up to the temporal arcade was performed in the remaining 33 eyes. Gas tamponade with 14% perfluoropropane or 18% sulfur hexafluoride was done in 19 eyes. Prednisolone acetate (1%; Pred Forte, Allergan, Irvine, CA, USA) and 0.5% levofloxacin (Cravit; Santen Pharmaceutical, Osaka, Japan), were topically instilled 4 times a day for 4 weeks.
After the surgery, patients were followed up at postoperative 1, 4, 8, and 14 months and then every 6 months or annually. All patients underwent a comprehensive ophthalmologic examination before and after the operation including measurement of BCVA, intraocular pressure (noncontact tonometer; KT-500, Kowa, Tokyo, Japan), slitlamp biomicroscopy, and indirect ophthalmoscopy. The refractive errors were measured by auto-refractometer (KR-8800; Topcon, Tokyo, Japan) and axial length by the IOL Master 500 (Carl Zeiss Meditec, Jena, Germany) before the surgery.
Spectral-domain optical coherence tomography images were obtained (Spectralis; Heidelberg Engineering, Heidelberg, Germany) with an eye-tracking system and automatic software to maintain the same position in serial scans. Central macular thickness (CMT) was measured as the distance between the first signal from the vitreoretinal interface and the outer border of the retinal pigment epithelium.
Myopic maculopathy was classified as category 0, no macular lesion; category 1, tessellated fundus; category 2, diffuse chorioretinal atrophy; category 3, patch chorioretinal atrophy; and category 4, macular atrophy with or without lacquer crack, choroidal neovascularization, and Fuchs spot as plus lesions according to the international classification and grading system for myopic maculopathy [20]. For logistic regression analysis, myopic maculopathy categories were divided into two groups, a group of categories 0, 1, 2 and a group of categories 3, 4.
Reviews of optical coherence tomography images and categorization of myopic maculopathy were performed by three investigators (CYK, MSK, and KLK) and inter-observer agreement was accomplished.
Statistical analyses were performed using IBM SPSS Statistics ver. 22 (IBM Corp., Armonk, NY, USA). Changes of BCVA and CMT at each postoperative follow-up were evaluated by paired t-test. Logistic regression analysis was performed to identify factors related with the functional and anatomical outcomes. Factors correlating with final BCVA were investigated by a linear regression model. A p-value less than 0.05 was considered statistically significant.
The demographics and preoperative clinical characteristics of the patients are presented in Table 1. Mean age was 62.2 ± 9.6 (range, 39.0 to 81.0) years and the patients were followed up for an average of 43.8 (range, 12.1 to 98.1) months after surgical treatment. Preoperative BCVA was 0.70 ± 0.44 logarithm of minimum angle of resolution (logMAR). Mean spherical equivalent was −8.90 ± 6.66 (range, +0.25 to −23.63) diopters and axial length was 30.52 ± 1.68 (range, 27.39 to 34.12) mm. Eighteen (45%) patients had previous cataract surgery and combined cataract surgery was performed in 13 (36%) patients. Eight (20%), 16 (40%), 8 (20%), and 8 (20%) eyes were classified with categories 1, 2, 3, and 4 myopic maculopathies, respectively.
Parameters evaluated in optical coherence tomography are listed in Table 1. Mean CMT was 526.6 ± 132.1 (range, 234.0 to 878.0) µm preoperatively. Twenty-four eyes (60%) had posterior vitreous detachment, 16 eyes (40%) had ERM, 40 eyes (100%) had foveoschisis and 6 eyes (27.5%) had FD, 32 eyes (80%) had ellipsoid zone (EZ) defect, and 21 eyes (52%) had interdigitation zone (IZ) defect in OCT.
Representative fundus photographs and serial OCT images at each postoperative follow-up are presented together with visual acuities (Fig. 1A–1H, 2A–2H, 3A–3H, 4A–4H). Postoperative BCVA and their changes are listed in Table 2. Mean BCVA improved from 0.70 ± 0.44 at baseline to 0.63 ± 0.57 logMAR at the final follow-up although it was not statistically significant (Fig. 5A, 5B). Preoperative CMT was 526.6 ± 132.1 µm and it continuously reduced in every follow-up with statistical significance (p < 0.001) (Table 2 and Fig. 5). At the final follow-up, it was 277.8 ± 92.1 µm, which was decreased by 248.8 ± 153.8 µm. Functional success was achieved in 24 (60%) eyes. Thirty-three (82.5%) eyes reached anatomical success and 7 (17.5%) failed. Among the failed cases, MH developed in 3 cases, MH retinal detachment in 2 cases, and recurrence of foveoschisis in 2 cases were documented. Seven cases were treated with repeated PPV with gas tamponade or silicone oil injection and 2 of them achieved functional success.
Factors associated with functional and anatomical outcome were identified using a logistic regression analysis. Longer axial length (odds ratio [OR], 1.60; 95% confidence interval [CI], 1.02 to 2.50; p = 0.041), higher category of myopic maculopathy (OR, 5.00; 95% CI, 1.27 to 19.69; p = 0.021), existence of FD (OR, 7.00; 95% CI, 1.48 to 33.21; p = 0.014) were significantly related with functional failure. In multivariate analysis, FD (OR, 6.53; 95% CI, 1.09 to 39.32; p = 0.041) showed an association with functional failure (Table 3).
In univariate analysis investigating factors related with anatomical outcome, higher category of myopic maculopathy (OR, 13.80; 95% CI, 1.47 to 130.1; p = 0.022) and FD (OR, 11.25; 95% CI, 1.75 to 72.50; p = 0.011) were found to be associated with anatomical failure. Multivariate analysis demonstrated significant association between FD and anatomical failure (OR, 8.01; 95% CI, 1.07 to 59.92; p = 0.043) (Table 4).
To find preoperative factors related with final BCVA, additional linear regression analysis was performed. Preoperative BCVA (r2 = 0.259, p = 0.001), axial length (r2 = 0.172, p = 0.008) and myopic maculopathy category (r2 = 0.336, p < 0.001) showed significant correlation with final BCVA (Fig. 6A–6C).
In this study, we investigated clinical factors related to the functional and anatomical outcomes of vitrectomy in MTM. The presence of FD and higher category of myopic maculopathy were associated with both functional and anatomical failure. Longer preoperative axial length showed an association with functional failure but not with anatomical failure. In addition, preoperative BCVA, axial length, and myopic maculopathy category showed significant correlation with final BCVA.
Functional and anatomical success was obtained in 60% and 82.5%, respectively. Previous studies on the surgical outcome of vitrectomy in MTM have reported visual acuity improvement in 42% to 100% and resolution of foveoschisis in 73% to 100% [9152122]. MH development was the most common complication of PPV for MTM. Regarding the rate of surgical success and complications, the results of our study were comparable to those of previous studies. Preoperative BCVA showed positive correlation with final BCVA and it was consistent with previous reports [141516]. Better preoperative BCVA implies a more preserved retinal neuronal function so greater visual rehabilitation can be expected after surgical treatment.
Kumagai et al. [14] reported that patients with FD had significantly better improvement of visual acuity and can benefit most from the surgery. However, our study showed a preoperative FD was a predictive factor for poor functional and anatomical outcomes. In the study by Kumagai et al. [14], patients were divided into two groups according to the presence of FD; those with FD showed greater visual acuity gain than those without FD. However, preoperative BCVA was worse in the patients with FD and other confounding factors were not controlled. In a recent study by Hattori et al. [19], eyes with FD showed worse pre- and postoperative BCVA compared to those without FD (p = 0.036, 0.046), although logMAR gain was not significantly different (p = 0.437). In our study, we did not divide subjects into sub-groups by the presence of FD but included suspected candidate factors in a multivariate logistic model. FD showed a significant correlation not only with functional but also anatomical outcome. The presence of FD should be considered for predicting the surgical outcome of MTM.
When EZ and IZ defects at final follow-up were analyzed, prevalence rates of EZ and IZ defects were both 100% in eyes with preoperative FD but 51.7% and 48.3% in eyes without preoperative FD, respectively. The final presentation of EZ and IZ defects was also significantly correlated with preoperative FD (p = 0.004, 0.003, respectively). It is speculated that the preoperative FD and disruption of photoreceptors affect final visual outcome. Further studies investigating the association between the duration, severity of FD, and surgical outcome would be helpful in clarifying the predictive ability of FD in MTM.
Previous studies have suggested preoperative factors such as baseline BCVA, axial length, photoreceptor defect, irregular choroidal surface, and FD were associated with surgical outcomes in MTM patients [14151617]. This study demonstrated that the eyes with a higher category of myopic maculopathy are less likely to achieve functional and anatomical success after vitrectomy. The severity of myopic degeneration is determined in part by the progressive distension of the posterior pole, therefore the structural destruction in the inner and outer retina by greater distension is estimated to induce more severe irreversible damages precluding visual recovery.
Among the 40 eyes, postoperative MH developed in 3 eyes (7.5%) and MH retinal detachment occurred in 2 eyes (5%) as postoperative complications. Preoperative BCVA (0.70 logMAR), CMT (529.2 µm), and the axial length (29.85 mm) of these eyes did not show significant difference compared with those without MH development. However, the prevalence of the EZ defect (100%) and FD (60%) was higher. Sayanagi et al. [18] reported higher prevalence of the EZ defect in FD and Gao et al. [13] demonstrated that the EZ defect is a risk factor for development of full-thickness MH after vitrectomy for myopic foveoschisis. The weakness of the inner retinal surface, combined with a disrupted inner segment/outer segment junction, increases its vulnerability to trauma during ILM peeling and contributes to MH development. The technical modification of ILM peeling, which spares foveal ILM was then introduced and demonstrated better anatomical and functional outcomes with decreased MH development [2324]. In our study, fovea-sparing ILM was performed in 7 eyes (18%) and none of them developed MH. Fovea-sparing ILM peeling thus may be one of the surgical options to reduce iatrogenic MH, especially in eyes with an EZ defect.
Our study has some limitations, mostly inherent in a retrospective study design. The sample size was small and follow-up period was variable. Due to the lack of standardized surgical indication for MTM, each surgeon has made surgical decisions individually and this was susceptible to selection biases.
In conclusion, a higher category of myopic maculopathy and the presence of FD are associated with functional and anatomical failure of PPV in MTM. Better preoperative BCVA, shorter axial length, and lower category of myopic maculopathy are associated with better final BCVA.
Figures and Tables
Fig. 1
A case of myopic traction maculopathy with foveal detachment after pars plana vitrectomy. (A) Preoperative and (B) final fundus images are presented. Serial optical coherence tomography images are arranged in chronological sequence: (C) preoperative, (D) 1 month, (E) 4 months, (F) 8 months, (G) 14 months, and (H) final. Visual acuities at each postoperative follow-up are indicated at the bottom right of the optical coherence tomography image.
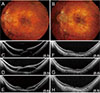
Fig. 2
A case of myopic traction maculopathy after pars plana vitrectomy with gas tamponade. (A) Preoperative and (B) final fundus images are presented. Serial optical coherence tomography images are arranged in chronological sequence: (C) preoperative, (D) 1 month, (E) 4 months, (F) 8 months, (G) 14 months, and (H) final. Visual acuities at each postoperative follow-up are indicated at the bottom right side of the optical coherence tomography image.
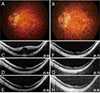
Fig. 3
A case of myopic traction maculopathy after pars plana vitrectomy without gas tamponade. (A) Preoperative and (B) final fundus images are presented. Serial optical coherence tomography images are arranged in chronological sequence: (C) preoperative, (D) 1 month, (E) 4 months, (F) 8 months, (G) 14 months, and (H) final. Visual acuities at each postoperative follow-up are indicated at the bottom right side of the optical coherence tomography image.

Fig. 4
A case of myopic traction maculopathy after pars plana vitrectomy with postoperative macular hole (*) development. (A) Preoperative and (B) final fundus images are presented. Serial optical coherence tomography images are arranged in chronological sequence: (C) preoperative, (D) 1 month, (E) 4 months, (F) 8 months, (G) 14 months, and (H) final. Visual acuities at each postoperative follow-up are indicated at the bottom right side of the optical coherence tomography image.

Fig. 5
Changes of (A) best-corrected visual acuity (BCVA) logarithm of minimum angle of resolution (logMAR) and (B) central macular thickness (CMT) at preoperative and different postoperative follow-up periods. *0.01 < p ≤ 0.05, **p ≤ 0.001.
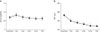
Fig. 6
Association between (A) preoperative best-corrected visual acuity (BCVA) logarithm of minimum angle of resolution (logMAR), (B) axial length (mm), (C) myopic maculopathy category and final BCVA (logMAR).

Table 2
Changes of BCVA and CMT after vitrectomy

Values are presented as mean ± standard deviation.
BCVA = best-corrected visual acuity; CMT = central macular thickness; logMAR = logarithm of the minimum angle of resolution; NA = not available.
*p-value of paired t-test between preoperative and postoperative BCVA; †p-value of paired t-test between preoperative and postoperative CMT.
References
1. Verkicharla PK, Ohno-Matsui K, Saw SM. Current and predicted demographics of high myopia and an update of its associated pathological changes. Ophthalmic Physiol Opt. 2015; 35:465–475.


2. Panozzo G, Mercanti A. Optical coherence tomography findings in myopic traction maculopathy. Arch Ophthalmol. 2004; 122:1455–1460.


3. Baba T, Ohno-Matsui K, Futagami S, et al. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am J Ophthalmol. 2003; 135:338–342.


4. Johnson MW. Myopic traction maculopathy: pathogenic mechanisms and surgical treatment. Retina. 2012; 32:S205–S210.
5. VanderBeek BL, Johnson MW. The diversity of traction mechanisms in myopic traction maculopathy. Am J Ophthalmol. 2012; 153:93–102.


6. Ikuno Y, Gomi F, Tano Y. Potent retinal arteriolar traction as a possible cause of myopic foveoschisis. Am J Ophthalmol. 2005; 139:462–467.


7. Panozzo G, Mercanti A. Vitrectomy for myopic traction maculopathy. Arch Ophthalmol. 2007; 125:767–772.


8. Shimada N, Tanaka Y, Tokoro T, Ohno-Matsui K. Natural course of myopic traction maculopathy and factors associated with progression or resolution. Am J Ophthalmol. 2013; 156:948–957.


9. Kobayashi H, Kishi S. Vitreous surgery for highly myopic eyes with foveal detachment and retinoschisis. Ophthalmology. 2003; 110:1702–1707.

10. Futagami S, Inoue M, Hirakata A. Removal of internal limiting membrane for recurrent myopic traction maculopathy. Clin Exp Ophthalmol. 2008; 36:782–785.


11. Kanda S, Uemura A, Sakamoto Y, Kita H. Vitrectomy with internal limiting membrane peeling for macular retinoschisis and retinal detachment without macular hole in highly myopic eyes. Am J Ophthalmol. 2003; 136:177–180.


12. Gaucher D, Haouchine B, Tadayoni R, et al. Long-term follow-up of high myopic foveoschisis: natural course and surgical outcome. Am J Ophthalmol. 2007; 143:455–462.


13. Gao X, Ikuno Y, Fujimoto S, Nishida K. Risk factors for development of full-thickness macular holes after pars plana vitrectomy for myopic foveoschisis. Am J Ophthalmol. 2013; 155:1021–1027.


14. Kumagai K, Furukawa M, Ogino N, Larson E. Factors correlated with postoperative visual acuity after vitrectomy and internal limiting membrane peeling for myopic foveoschisis. Retina. 2010; 30:874–880.


15. Hwang JU, Joe SG, Lee JY, et al. Microincision vitrectomy surgery for myopic foveoschisis. Br J Ophthalmol. 2013; 97:879–884.


16. Figueroa MS, Ruiz-Moreno JM, Gonzalez del, et al. Long-term outcomes of 23-gauge pars plana vitrectomy with internal limiting membrane peeling and gas tamponade for myopic traction maculopathy: A Prospective Study. Retina. 2015; 35:1836–1843.

17. Shin JY, Yu HG. Visual prognosis and spectral-domain optical coherence tomography findings of myopic foveoschisis surgery using 25-gauge transconjunctival sutureless vitrectomy. Retina. 2012; 32:486–492.


18. Sayanagi K, Ikuno Y, Soga K, Tano Y. Photoreceptor inner and outer segment defects in myopic foveoschisis. Am J Ophthalmol. 2008; 145:902–908.


19. Hattori K, Kataoka K, Takeuchi J, et al. Predictive factors of surgical outcomes in vitrectomy for myopic traction maculopathy. Retina. 2018; 38:S23–S30.

20. Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015; 159:877–883.


21. Taniuchi S, Hirakata A, Itoh Y, et al. Vitrectomy with or without internal limiting membrane peeling for each stage of myopic traction maculopathy. Retina. 2013; 33:2018–2025.


22. Ikuno Y, Sayanagi K, Ohji M, et al. Vitrectomy and internal limiting membrane peeling for myopic foveoschisis. Am J Ophthalmol. 2004; 137:719–724.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download