Abstract
Purpose
We sought to investigate the effects of Graves' orbitopathy (GO) and orbital decompression on lamina cribrosa depth (LCD) using spectral-domain optical coherence tomography.
Methods
Forty eyes that underwent orbital decompression to relieve compressive optic neuropathy or correct disfiguring exophthalmos in the context of GO were included. Subjects were imaged with spectral-domain optical coherence tomography before surgery and at 1 and 3 months after surgery, at which the examiner measured the LCD (distance from the anterior surface of the lamina cribrosa to the Bruch membrane opening line) and peripapillary retinal nerve fiber layer thickness. Subjects were divided into two groups—a muscle-dominant group composed of patients who had extraocular muscle enlargement on preoperative orbital computed tomography scan and a fat-dominant group composed of patients who did not show extraocular muscle enlargement on preoperative orbital computed tomography scan—and subgroup analysis was performed. Preoperative and postoperative intraocular pressure, exophthalmos, LCD, and retinal nerve fiber layer thickness were evaluated.
Results
At baseline, LCD was remarkably shallower in the muscle-dominant group than in the fat-dominant group (95% confidence interval, p = 0.007). In the muscle-dominant group, LCD showed no definite change after surgery. However, the fat-dominant group showed temporary posterior displacement of the lamina cribrosa at 1-month postoperation that was reversed to baseline at 3 months postoperation (95% confidence interval, p < 0.01).
Conclusions
The lamina cribrosa was anteriorly displaced preoperatively, and its position was nearly unchanged after the surgery, especially in association with extraocular muscle enlargement. An enlarged extraocular muscle could reduce the pressure-relieving effect of orbital decompression around the scleral canal in patients with GO.
Graves' orbitopathy (GO) is an autoimmune disorder characterized by expansion of retrobulbar tissue including fat and extraocular muscle, leading to several sequelae such as proptosis, strabismus, eyelid abnormality, and decreased visual acuity [1]. Orbital decompression has been previously regarded as a suitable treatment of choice to minimize such sequelae in patients with GO [2]. This approach involves the removal of bone or fatty tissue composing the orbit, resulting in a radical change in orbit anatomy [3]. Orbital decompression may also improve orbital vascular circulation to avoid sight-threatening complications with respect to compressive optic neuropathy (CON) [4].
The lamina cribrosa (LC) is the structure that supports the optic nerve axons and the central retinal artery that passes through the lamina pores. Posterior displacement of the LC has been regarded as one of the important causes of glaucomatous optic nerve damage due to compromised axonal transport and blood flow to the optic nerve [5]. After intraocular pressure (IOP)-lowering surgery, reversal of LC displacement was observed in patients with primary open-angle glaucoma [6]. The LC position is determined by translaminar pressure difference, or the difference between IOP and retrolaminar tissue pressure [7]. Dev et al. [8] observed that IOP decreased after orbital decompression for GO. In addition, Otto et al. [9] demonstrated that retrobulbar pressure decreased after orbital decompression and Perez-Lopez et al. [10] evaluated the changes in retrobulbar ocular blood flow in conjunction with orbital decompression and showed that resistance indexes in the central retinal artery and ophthalmic artery decreased after the procedure. Further, patients with GO may have abnormal choroidal perfusion, and orbital decompression may improve choroidal circulation in these individuals [4]. In view of these previous studies, we evaluated baseline LC depth (LCD) and change in LCD after orbital decompression in GO.
This prospective, observational case series study was conducted at Severance Hospital, Yonsei University College of Medicine in Seoul, Korea. The sample size calculation was based on LCD change from baseline to the 3-month follow-up between a muscle-dominant group and a fat-dominant group. Assuming a 90% power with a type I error rate of 0.05, the study would need to recruit more than 36 eyes in total.
Sixty-nine eyes of 42 patients with GO who received orbital two-wall decompression with fat or bone removal were enrolled in the study. Patients underwent surgery to correct disfiguring proptosis caused by GO or to relieve CON. All decompression surgeries in this study were performed by one surgeon with the same surgical method. In this case series, surgical methods were not significantly different between the muscle- and fat-dominant groups. About 3 to 4 mL of intraconal fat was removed selectively from the posterior and temporal orbital areas in both groups. All patients underwent orbital two-wall decompression of the ethmoid and floor with posterior strut removal. Lateral decompression was not performed. The surgical procedure was mostly standardized, although the amount of decompression varied according to preoperative degree of exophthalmos. Patients who had history of inflammatory disease, ocular surgery, or trauma were excluded. Eyes with coexisting retinal or glaucomatous disease that could affect the retinal nerve fiber layer (RNFL) thickness also were excluded from this study. The institutional review board of Severance Hospital, Yonsei University College of Medicine approved this study (IRB 4-2017-0582). This study followed the tenets of the Declaration of Helsinki, and written informed consent was obtained from all participants after an explanation of the nature and possible consequences of the study.
Clinical data of age, sex, spherical equivalent (measured with the KR-7100 autokeratometer; Topcon, Tokyo, Japan), axial length (measured using the IOLMaster; Carl Zeiss Meditec, Dublin, CA, USA), central corneal thickness (measured using the UP-1000 ultrasonic pachymeter; Nidek Technologies, Gamagori, Japan), preoperative IOP as measured by Goldmann applanation tonometry, and the Hertel exophthalmometry value were collected for each subject. Optic discs were examined using the Spectralis optical coherence tomography (OCT) system (Heidelberg Engineering GmbH, Heidelberg, Germany) at 1 day before surgery and at 1 and 3 months after surgery. IOP and Hertel exophthalmometry values were evaluated at 3 months after surgery.
A previous study showed that the enhanced depth imaging
technique could visualize the anterior surface of the LC better than the conventional imaging technique [11]. The details of this technique have been described in a previous study [11]. Patients were imaged through dilated pupils using a 10- × 15-degree rectangle covering the optic disc. This rectangle was scanned with approximately 80 to 90 sections, which were 30 µm apart with an average of 42 OCT frames.
LCD was measured using a previously described method on B-scan images preoperatively and at 1 and 3 months postoperatively [6]. The LCD value was determined by measuring the distance from the Bruch membrane opening (BMO) plane to the level of the anterior LC surface. The anterior surface of the LC was defined by the highly reflective structure below the optic cup. A reference line connecting the two termination points of the Bruch membrane was drawn on each B-scan image. The distance from the reference line to the level of the anterior border of the LC was then measured at three points: the maximally depressed point and two additional points located 100 µm from the maximally depressed point in the temporal and nasal directions, respectively. The distance was measured on the line perpendicular to the reference line. All measurements were performed using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD, USA; available in the public domain at http://rsb.info.nih.gov/ij/index.html). The average value of these three points was considered the LCD for each selected B-scan image. Nine horizontal B-scan images (mid-horizontal center, four scans superior to center, and four scans inferior to center) were estimated, and values from each image were averaged. When the anterior border of the LC was not well-visualized on specific B-scan images, an adjacent image was used for measurements. If more than three among the nine B-scan images were not well-visualized to discriminate the anterior surface of the LC, the subject was excluded from this study. All the measurements were performed by one observer (YRS) who was blinded to the clinical information of each patient. To evaluate the interobserver reproducibility of the measurements, 30 randomly selected B-scan images were evaluated by two independent examiners (HWB and JSY). The intraclass correlation coefficient (ICC) was 0.81.
Prelaminar tissue thickness was calculated by measuring additional values. Perpendicular distances were measured from the reference line to the surface of the optic cup at the three points where the LCD was determined. The prelaminar tissue thickness was defined as the difference between the distance from the reference line to the optic cup surface and that from the reference to the anterior LC surface [6]. It was also measured in the same manner as the LCD. The diameter of the BMO was determined at the same mid-horizontal plane of the optic nerve head in both preoperative and postoperative images.
The ICC value was 0.78 for the prelaminar tissue and 0.83 for the BMO, which were calculated using the same method as for LCD measurement.
An A-scan was performed for measuring peripapillary RNFL thickness using an A-scan circle whose diameter was 3.45 mm and that was positioned manually at the center of the optic disc. The Heidelberg Eye Explorer (viewing module of the Spectralis OCT system) calculated the global average RNFL thickness for the overall 360 degrees of the circle.
In a previous study, compression by enlarged muscle at the orbital apex was regarded as the mechanism involved in damage to the optic nerve [12]. To evaluate the effect of enlargement of the extraocular muscle on the optic nerve, we divided subjects into two groups depending on extraocular enlargement on computed tomography (CT) scan before the surgery. The muscle-dominant group included subjects who had extraocular muscle enlargement in the preoperative CT scan, while the fat-dominant group included subjects who did not have extraocular muscle enlargement in the preoperative CT scan. Axial and coronal images in the CT scans were evaluated for muscle enlargement. The image slice that included the lens bilaterally in the axial cut and the image in which the vertical muscle diameters were maximal in the coronal cut among the CT scans were used to group fat- and muscle-dominant patients. The extraocular muscle was designated as enlarged if it was larger than the optic nerve on the image [1314]. Clinical variables (age, sex, spherical equivalent, axial length, central corneal thickness, preoperative and postoperative IOP, preoperative exophthalmos, degree of exophthalmos reduction) were compared between the two groups. Changes in LCD and peripapillary RNFL thickness were compared between the preoperative and postoperative periods.
The LCD in the preoperative and postoperative periods, the thickness of the prelaminar tissue, and the mid-horizontal BMO diameter were compared using repeated-measures analysis of variance. Preoperative and postoperative exophthalmos and IOP change were analyzed by paired t-tests. Fisher's exact test and independent t-test were appropriately used for comparing clinical variables in the subgroup analysis after normality was verified. Normality tests for LCD, thickness of the prelaminar tissue, and BMO diameter were performed with the Kolmogorov-Smirnov test, and all parameters were normal (LCD, p = 0.1; thickness of prelaminar tissue, p = 0.08; BMO diameter, p = 0.17). Statistical analysis was performed using SPSS Statistics ver. 17.0 (SPSS Inc., Chicago, IL, USA). A p-value less than 0.05 was considered significant.
Among 69 eyes enrolled in this study, 40 were included after excluding 19 whose OCT image quality was too poor to identify the surface of the LC (greater than three missing B-scan images) and 10 who did not complete all follow-up imaging. The mean age was 36.83 ± 2.15 years (range, 18 to 71 years). Twelve subjects were men and 28 subjects were women. The mean spherical equivalent was −2.62 ± 0.43 diopters (range, 0.94 to 9.12 diopters). The mean central corneal thickness was 541.47 ± 6.26 µm (range, 486 to 604 µm). The mean axial length was 24.77 ± 0.21 mm (range, 21.98 to 27.1 mm). Preoperative IOP was 15.1 ± 0.53 mmHg (range, 9 to 25 mmHg). The mean preoperative exophthalmos value was 20.95 ± 2.51 mm (range, 16 to 26 mm). Since the first diagnosis, the mean duration of thyroid dysfunction was 99.40 ± 14.95 months, and the mean duration of GO was 63.50 ± 6.13 months (Table 1).
The degree of exophthalmos markedly decreased from 20.95 ± 2.51 to 15.8 ± 2.27 mm after orbital decompression (p < 0.001). IOP also decreased significantly from 15.1 ± 0.53 to 14.13 ± 3.27 mmHg after surgery (p < 0.001) (Table 2).
The ICC values for evaluation of BMO, LCD, and prelaminar tissue were 0.908, 0.863, and 0.825, respectively. The amount of LCD was 561.91 ± 31.7 µm preoperatively and 629.45 ± 34.66 and 584.78 ± 33.98 µm at postoperative 1 and 3 months, respectively. The LC showed marked posterior displacement at 1 month postoperation (preoperation vs. 1 month postoperation, p = 0.001). However, its displacement was significantly reversed to the preoperative level at 3 months postoperation (preoperation vs. 3 months postoperation, p = 0.117; 1 vs. 3 months postoperation, p = 0.004). The BMO sizes were 1,694.37 ± 31.91, 1,704.9 ± 33.76, and 1,672.63 ± 34.18 µm before surgery and at 1 and 3 months after surgery, respectively. The size of the BMO increased at 1 month postoperation and then decreased significantly at 3 months postoperation (preoperation vs. 1 month postoperation, p = 0.268; 1 vs. 3 months postoperation, p < 0.001; preoperation vs. 3 months postoperation, p = 0.26). Thickness of prelaminar tissue was 280.67 ± 22.38, 308.33 ± 26.91, and 280.74 ± 23.2 µm at preoperation, 1 month postoperation, and 3 months postoperation, respectively. Prelaminar tissue thickness experienced no significant change following surgery (preoperation vs. 3 months postoperation, p = 0.99) (Fig. 1A). Peripapillary RNFL thickness was measured at preoperation and 3 months postoperation. It also showed no definite change after surgery (98.55 ± 12.76 vs. 99.98 ± 12.34 µm, p = 0.1)
In subgroup analysis, the muscle-dominant group (subjects whose muscle enlargement was confirmed on preoperative CT) included 15 eyes, while the fat-dominant group (subjects whose muscle enlargement was not identified during preoperative CT) included 25 eyes. All clinical variables other than age showed no significant difference. The degree of exophthalmos reduction after surgery showed no significant difference between the two groups (muscle-dominant group, 5.36 ± 2.01 mm vs. fat-dominant group, 5.02 ± 1.74 mm; p = 0.57) (Table 3).
At baseline, LCD was remarkably shallower in the muscle-dominant group than in the fat-dominant group (muscle-dominant group, 462.79 ± 95.96 µm vs. fat-dominant group, 621.39 ± 78.39 µm; p = 0.007). After surgery, in the fat-dominant group, the LC was displaced posteriorly at 1 month postoperation and reversed at 3 months postoperation (LCD at preoperative period, 621.39 ± 78.39 µm; 1 month postoperation, 709.94 ± 82.31 µm; 3 months postoperation, 640.92 ± 74.91 µm; preoperation vs. 1 month postoperation, p < 0.01; preoperation vs. 3 months postoperation, p = 1.0; 1 vs. 3 months postoperation, p < 0.01). However, LCD showed no definite change during the follow-up period in the muscle-dominant group (LC displacement preoperation, 462.79 ± 95.96 µm; 1 month postoperation, 495.3 ± 81.19 µm; 3 months postoperation, 491.19 ± 104.2 µm; preoperation vs. 1 month postoperation, p = 0.53; preoperation vs. 3 months postoperation, p = 0.4; 1 vs. 3 months postoperation, p = 1.0). In repeated-measures analysis of variance, the two groups showed a significant difference according to preoperative extraocular muscle enlargement (p = 0.007) (Fig. 1B, 1C). Peripapillary RNFL thickness showed no definite change after surgery in either group (muscle-dominant group, 98.38 ± 5.29 to 99.46 ± 4.9 µm vs. fat-dominant group, 101.32 ± 102.4 to 102.4 ± 12.86 µm; p = 0.14) and no definite difference between the two groups (p = 0.3).
Two subjects with CON were included in the muscle-dominant group. Demographic data and LCD change are described in Table 4 and Fig. 2. In both subjects, LCD change was not remarkable. The LCD values for subject 1 were 346.67 µm (preoperation), 348.50 µm (1 month postoperation), and 355.17 µm (3 months postoperation). Those for subject 2 were 254.27 µm (preoperation), 253.67 µm (1 month postoperation), and 251.60 µm (3 months postoperation) (Fig. 2).
To our knowledge, this is the first report to evaluate microstructural changes in the optic nerve rather than the macrostructure of orbit anatomy after orbital decompression. The mean baseline LCD was shallower with extraocular muscle enlargement than when it was absent. Additionally, in the muscle-dominant group, LCD showed no definite change after surgery, contrary to the fat-dominant group.
According to previous normal data in which the LCD was evaluated depending on the mean axial length of the eyeball, the mean LCD was 578.2 ± 163.5 µm when the axial length fell in the range of 23 to 26 mm [15]. Even though the results of the present study could not be directly compared with these previous data, the LC of the muscle-dominant group was displaced more anteriorly than that of the fat-dominant group at baseline. Further, the LCD of the muscle-dominant group at baseline tended to be shallower than the previous normal value. Considering increased retrobulbar pressure in patients with GO [9], and that the IOP of enrolled patients was not elevated compared with the normal value, translaminar pressure difference, determined as the difference between IOP and retrobulbar pressure, may move to the anterior surface of the LC in the muscle-dominant group. Compared to the fat-dominant group, the extraocular muscle was enlarged in the limited orbit space, and the stiffness of orbital tissue increased in the muscle-dominant group [16]. Those characters of extraocular muscle could increase the retrobulbar pressure in the muscle-dominant group, leading to relatively anterior displacement of the LC (Fig. 3A, 3B).
After surgery, the LC sustained its depth in the muscle-dominant group, contrary to the temporary posterior displacement and reversal of the LC in the fat-dominant group, although similar amounts of exophthalmos reduction and IOP change were observed in the two groups (Fig. 1C). The primary event of GO in the muscle-dominant group is muscle enlargement. Therefore, anterior displacement of LC in the muscle-dominant group is believed to be related to the “stiffness and rigidity of extraocular muscle” surrounding the optic nerve and eyeball [1718]. The minimal change of LCD after orbital decompression in the muscle-dominant group was due to the “low resilience” of muscle, which even becomes more severe after orbital decompression [19].
On the contrary, in the fat-dominant group, more posterior LC displacement developed at 1 month after surgery and was restored to the baseline position at 3 months after surgery. As it is impossible to directly measure the change of retrobulbar pressure, the precise mechanism for this serial change of LC depth after surgery cannot be verified. However, the change of LCD after surgery seems to be related to the abrupt disequilibrium and slow recovery of the IOP-retrobulbar pressure relationship. The abrupt pressure change could have been induced by herniation of stretched and relaxed muscles into the expanded bony space. Over time, wound healing of the periorbita and soft tissues of the decompressed orbit might have caused a variable degree of fibrosis.
To find an appropriate explanation, representative preoperative and postoperative 3-month CT scans of a patient with fat-dominant proptosis were reviewed (Fig. 4A, 4B). As we see in preoperative CT scans, the redundant optic nerve straightens and stretches in response to intraconal fat expansion, resulting in axial proptosis. Probably in this period, simultaneous with the globe being pushed forward by fat volume, the optic nerve and muscles underwent adaptation and lengthening due to stretching, pulling the LC backward. On the postoperative CT scan at 1 month after surgery, the extraocular muscles and optic nerve were still elongated and flexible. An especially long, thin, elastic inferior rectus muscle was herniated through the maxillary sinus. This could cause sudden reduction in optic nerve tension and intraconal pressure, resulting in more posterior displacement, albeit transient for LC. However, in CT scan images at 3 months after surgery, the extraocular muscles were enlarged, showing gain in elasticity and decrease in intraconal fat volume. Although the configuration of the optic nerve was not precisely defined in CT scans, the optic nerve probably readapted and achieved elasticity according to the reconformation of orbital volume.
Consequently, the LC maintained its depth in the muscle-dominant group despite the surgery. Two subjects with CON were included in the muscle-dominant group in this study (Fig. 2). Their baseline LC positioning (346.67 and 254.27 µm) was anteriorly displaced compared to previous normal values (578.2 ± 163.5 µm). This result represents evidence that enlarged extraocular muscle increase retrobulbar pressure [9], causing anterior displacement of the LC. In addition, these two subjects showed no definite change in LC position during follow-up. This could be explained by the stiffness of the extraocular muscle mentioned above.
The RNFL thickness was measured at 3 months postoperation and demonstrated no definite change compared to the preoperative level. Given that visual field loss was preceded by RNFL loss, and that progressive disc change may be detected before visual field change [202122], the study showed that visual field damage was not present at 3 months postoperation. However, RNFL thinning progressed even 2.5 years after trabeculectomy, which induced anterior displacement of the LC in patients with primary-open angle glaucoma in previous research [2324]. Considering these prior studies, the follow-up period of this study was too short to determine the effect of orbital decompression on RNFL thickness. Accordingly, continuous follow-up is needed for evaluating RNFL damage after orbital decompression, which induces LCD change such as that seen in the fat-dominant group. If RNFL damage would be observed in this group, appropriate action (e.g., IOP-lowering drugs) should be performed for preventing additional damage.
This study has some limitations. First, it included a small number of subjects because of a difficulty in acquiring good-quality images and sustained follow-up data. Second, we could not perform comparisons with age- and sex-matched normal controls or patients with GO who did not receive surgery. Third, the follow-up period was too short to measure the change in RNFL thickness caused by orbital decompression. Furthermore, we could not include visual function testing results, as they were not sufficient to analyze. Also, we did not analyze the LCD according to axial length, although baseline axial length showed no definite difference between the two groups. We hope to continue analyses of LCD in both GO and normal control groups and compare the data.
Despite these limitations, we demonstrated the sheer mechanical effect of orbital decompression on the microstructure of the optic nerve in this study. The results of this study suggest that extraocular muscle enlargement is important for lowering the decompressive effect of orbital decompression around the scleral canal. Further study is required to determine the effect of GO itself comparing age- and sex-matched normal controls and that of orbital decompression on optic disc microstructures with a larger sample of patients with CON.
Figures and Tables
Fig. 1
Microstructural changes of orbital anatomy after orbital decompression surgery. (A) Change in mean magnitude of lamina cribrosa depth (LCD), prelaminar tissue thickness, and Bruch membrane opening (BMO) at each follow-up visit (preoperation and 1 and 3 months postoperation) (analyzed by repeated-measures ANOVA with Bonferroni method for multiple comparison, *p < 0.05). (B) Comparison of LCD change between the muscle-dominant group and fat-dominant group. In the muscle-dominant group, the position of the lamina cribrosa showed no definite change in the postoperative periods compared with at baseline. In the fat-dominant group, the position of the lamina cribrosa was posteriorly displaced at 1 month postoperation and reversed at 3 months postoperation. (C) Representative images for comparison of LCD change between the muscle-dominant group and fat-dominant group. B-scan images were obtained preoperatively and at 1 and 3 months postoperatively from patients who underwent orbital decompression. A reference line was set by connecting the termination of Bruch's membrane (yellow solid line). Green solid line: B-scan-acquired site in optic nerve head.

Fig. 2
Lamina cribrosa (LC) depth change in subjects diagnosed with compressive optic neuropathy due to Graves' orbitopathy before orbital decompression.

Fig. 3
Schematic illustration of the change in lamina cribrosa depth after orbital decompression, comparing the fat-dominant group and the muscle-dominant group. (A) In the fat-dominant group, at baseline, retrobulbar pressure (RBP) was lower than that of the muscle-dominant group. Therefore, the translaminar pressure difference moves to the posterior surface of the lamina cribrosa. After orbital decompression, the RBP decreased, and the net translaminar pressure difference increased toward the same direction. (B) In the muscle-dominant group, at baseline, the RBP was relatively larger than that of the fat-dominant group, and the translaminar pressure difference was directed toward the anterior surface of the lamina cribrosa. After surgery, intraocular pressure (IOP) and RBP decreased slightly at the same time. Therefore, there was no definite change in translaminar pressure difference. Cerebrospinal fluid pressure is not indicated in this diagram under the assumption that it did not change after the surgery.
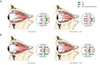
Fig. 4
Change of intraorbital structure in patients of two groups (A, fat-dominant group; B, muscle-dominant group) between the preoperative and postoperative periods on orbital computed tomography scans. (A) In the fat-dominant group, extraocular muscle and optic nerve seemed to lose their tension temporarily at 1 month after surgery but was restored at 3 months after surgery. (B) In contrast, in the muscle-dominant group, there was only a slight change in tension of the extraocular muscle and optic nerve compared to the fat-dominant group.

Table 3
Comparison of clinical demographics between the muscle-dominant group and fat-dominant group

Values are presented as mean ± standard deviation or number (%); LC displacement change (1 month postoperation) = postoperative 1 month displacement − preoperative LC displacement; LC displacement change (3 months postoperation) = postoperative 3 month displacement − preoperative LC displacement.
D = diopters; CCT = central corneal thickness; IOP = intraocular pressure; LC = lamina cribrosa.
*Analyzed by independent t-test after normality test (Shapiro-Wilk test, all p > 0.05); †p < 0.05; ‡Analyzed by Fisher's exact test.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2017R1A2B4009565). The funding organization had no role in the design or conduct of this research.
The authors would like to thank Dong-Su Jang, MFA (Medical Illustrator) for his help with the illustrations.
References
2. Dolman PJ. Evaluating Graves' orbitopathy. Best Pract Res Clin Endocrinol Metab. 2012; 26:229–248.

3. Garrity JA, Fatourechi V, Bergstralh EJ, et al. Results of transantral orbital decompression in 428 patients with severe Graves' ophthalmopathy. Am J Ophthalmol. 1993; 116:533–547.

4. Nik N, Fong A, Derdzakyan M, et al. Changes in choroidal perfusion after orbital decompression surgery for Graves' ophthalmopathy. J Ophthalmic Vis Res. 2017; 12:183–186.
5. Burgoyne CF, Downs JC, Bellezza AJ, et al. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005; 24:39–73.

6. Lee EJ, Kim TW, Weinreb RN. Reversal of lamina cribrosa displacement and thickness after trabeculectomy in glaucoma. Ophthalmology. 2012; 119:1359–1366.

7. Burgoyne CF. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 2011; 93:120–132.

8. Dev S, Damji KF, DeBacker CM, et al. Decrease in intraocular pressure after orbital decompression for thyroid orbitopathy. Can J Ophthalmol. 1998; 33:314–319.
9. Otto AJ, Koornneef L, Mourits MP, Deen-van Leeuwen L. Retrobulbar pressures measured during surgical decompression of the orbit. Br J Ophthalmol. 1996; 80:1042–1045.

10. Perez-Lopez M, Sales-Sanz M, Rebolleda G, et al. Retrobulbar ocular blood flow changes after orbital decompression in Graves' ophthalmopathy measured by color Doppler imaging. Invest Ophthalmol Vis Sci. 2011; 52:5612–5617.
11. Girard MJ, Tun TA, Husain R, et al. Lamina cribrosa visibility using optical coherence tomography: comparison of devices and effects of image enhancement techniques. Invest Ophthalmol Vis Sci. 2015; 56:865–874.

12. Neigel JM, Rootman J, Belkin RI, et al. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology. 1988; 95:1515–1521.
13. Ben Simon GJ, Syed HM, Douglas R, et al. Extraocular muscle enlargement with tendon involvement in thyroid-associated orbitopathy. Am J Ophthalmol. 2004; 137:1145–1147.

14. Ozgen A, Ariyurek M. Normative measurements of orbital structures using CT. AJR Am J Roentgenol. 1998; 170:1093–1096.

15. Yun SC, Hahn IK, Sung KR, et al. Lamina cribrosa depth according to the level of axial length in normal and glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2015; 253:2247–2253.

16. Wilson WB, Manke WF. Orbital decompression in Graves' disease. The predictability of reduction of proptosis. Arch Ophthalmol. 1991; 109:343–345.
17. Kennerdell JS, Rosenbaum AE, El-Hoshy MH. Apical optic nerve compression of dysthyroid optic neuropathy on computed tomography. Arch Ophthalmol. 1981; 99:807–809.

18. Trokel SL, Jakobiec FA. Correlation of CT scanning and pathologic features of ophthalmic Graves' disease. Ophthalmology. 1981; 88:553–564.

19. Kim JW, Lee KH, Woo YJ, et al. The effect of systemic steroids and orbital radiation for active Graves orbitopathy on postdecompression extraocular muscle volume. Am J Ophthalmol. 2016; 171:11–17.

20. Motolko M, Drance SM. Features of the optic disc in preglaucomatous eyes. Arch Ophthalmol. 1981; 99:1992–1994.

21. Pederson JE, Anderson DR. The mode of progressive disc cupping in ocular hypertension and glaucoma. Arch Ophthalmol. 1980; 98:490–495.

22. Sommer A, Pollack I, Maumenee AE. Optic disc parameters and onset of glaucomatous field loss. I. Methods and progressive changes in disc morphology. Arch Ophthalmol. 1979; 97:1444–1448.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download