Abstract
Purpose
To evaluate the changes in visual acuity (VA) and central macular thickness (CMT) after intravitreal dexamethasone (IVD) implantation in intravitreal bevacizumab (IVB) treatment-resistant cases with pseudophakic cystoid macular edema (PCME).
Methods
This study included 10 PCME cases who underwent uneventful phacoemulsification and intraocular lens implantation with similar methods and six PCME cases referred to our hospital for treatment of low VA after cataract surgery. Due to the persistence of PCME, both topical steroid and anti-inflammatory medication were administered first, followed by IVB injection. IVD implantation was performed for all IVB treatment-resistant cases. VA and CMT values were compared before and at three months after the first IVD implantation.
Results
The mean VA values before and at 3 months after the first IVD implantation were 0.69 ± 0.19 logarithm of the minimum angle of resolution (logMAR) (1.50 to 0.10 logMAR) and 0.26 ± 0.07 logMAR (1.00 to 0.00 logMAR), respectively (p < 0.001). The mean CMT was 476.13 ± 135.13 mm (314 to 750 mm) and 294.06 ± 15.26 mm (222 to 480 mm), respectively (p < 0.001). The mean number of implanted IVD was 1.44 ± 0.89 (1 to 4) and the mean follow-up time was 7.4 ± 4.6 months (6 to 24 months). After IVD implantation therapy, the mean VA and CMT values were 0.19 ± 0.05 logMAR (0.70 to 0.00 logMAR) and 268.38 ± 31.35 mm (217 to 351 mm), respectively.
Conclusions
To the best of our knowledge, this is the first report to show the efficacy of IVD implantation even after repeated IVB injections in treatment-resistant PCME. IVD implantation is both a safe and effective method for decreasing PCME after both uneventful and complicated cataract surgery.
Pseudophakic cystoid macular edema (PCME) (Irvine-Gass syndrome) is a common cause of a painless decrease in visual acuity (VA) following cataract surgery [1]. PCME can occur even after uneventful surgery, and the pathogenesis is based predominantly on inflammation, vascular instability, vitreomacular traction, and light toxicity [234]. Some pathologies such as vasculopathy, hypertension, and uveitis are predisposing factors for the development of PCME, and the incidence of PCME is higher in patients with these diseases [567]. Optical coherence tomography (OCT) is widely used for diagnosing PCME as it clearly demonstrates the presence and nature of perifoveal cystic spaces [8].
PCME is usually self-limiting and spontaneous resolution can occur within a few months in many cases [9]. However, in some cases, PCME persists for more than six months and is then considered chronic [1011]. Although medical therapies are usually applied, there is still no widely accepted consensus on the efficacy of various therapeutic options. Topical non-steroidal anti-inflammatory drugs and topical/periocular corticosteroids may be efficacious in reducing PCME. Although the dominant pathogenesis in the development of PCME is inflammation, intravitreal bevacizumab (IVB) and other anti-vascular endothelial growth factor agents are important treatment options that can inhibit the development of PCME, as vascular endothelial growth factor leads to an increase in vascular permeability [12]. Sustained-release intravitreal dexamethasone (IVD) implants can be effective in treatment-resistant cases through their highly potent anti-inflammatory and anti-edema effects [1314].
In this study, we evaluated the functional and anatomic response to IVD implantation by comparing VA and central macular thickness (CMT) before and after IVD implantation therapy in IVB treatment-resistant cases with PCME.
This retrospective, case series study was conducted by the department of ophthalmology of a referral university hospital, after obtaining approval from the Ministry of Health Review Board (75642246-518.01-78994). A written infromed consent was obtained from each patient. All procedures were performed in accordance with the ethical standards of the Helsinki Declaration for human subjects.
We retrospectively examined the medical documents of IVB (Avastin; Genentech, South San Francisco, CA, USA) treatment-resistant adult patients with PCME after phacoemulsification and intraocular lens (IOL) implantation between 2012 and 2017. Detailed medical history, ocular examination, ophthalmological ancillary tests, and routine laboratory results were present in the medical records. Patients with a history of any type of uveitis, anterior or posterior segment surgery, retinal artery or vein occlusion, patients with preoperative vitreomacular interface diseases, those taking topical prostaglandin analog medication for glaucoma, those with complications after cataract surgery (such as endophthalmitis or retained cortical material), diabetic retinopathy, hypertensive retinopathy, and eyes with choroidal neovascular membrane were excluded from this study.
In 10 cases, phacoemulsification and IOL implantation were performed with similar methods by a single experienced surgeon (AKA). Six additional cases were referred to our hospital for treatment of low VA after cataract surgery.
In the 10 cases treated at our hospital, topical tropicamide 1% and proparacaine 0.5% was administered for pupil dilation and anesthesia. A triplanar main incision and side port incisions were made then 0.1 mL adrenalin 0.001% and 0.1 mL aritmal 2% were injected. Ophthalmic viscoelastic devices were used with the soft-shell method. Phacoemulsification was performed with the Constellation Vision System (Alcon, Fort Worth, TX, USA). The divide and conquer technique was used for nucleus fragmentation and phacoemulsification. Low vacuum and ultrasonic power parameters were set for nucleofractis and irrigation/aspiration. All 10 patients had uneventful surgery and posterior chamber in the bag AcrySof IQ monofocal IOLs (Alcon) were implanted. After intracameral antibiotic and subconjunctival steroid injection, surgery was finalized. Topical moxifloxacin (1 week 3 drops per day) and topical dexamethasone 0.1% (1 month, 3 drops per day) were prescribed as standard postoperative care. No postoperative complications, such as corneal edema, anterior chamber reaction, or increased intraocular pressure, developed in any patients.
In four of the six patients referred to our hospital, cataract surgeries were complicated by posterior capsular rupture, and a 3-piece posterior chamber ciliary sulcus fixated IOL was implanted (Tecnis 3-Piece; Abbot Medical Optics, Santa Ana, CA, USA). Posterior chamber in the bag AcrySof IQ monofocal IOL (Alcon) was successfully implanted in the other two patients.
The six patients referred from other hospitals and the 10 patients operated on in our hospital who had VA that only slightly increased in the early postoperative period or decreased one month postoperatively after a significant early increment underwent a detailed ophthalmological evaluation to investigate the cause of low VA. PCME was diagnosed based on symptoms including painless visual loss or absence of visual improvement after surgery, ocular examinations including best corrected VA, slit-lamp biomicroscopy and fundoscopy after pupillary dilation, and spectral-domain OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany) evaluation, which revealed the increase of CMT without associated permanent fibrotic or atrophic changes. PCME had persisted for a minimum of three months, and during this period, all patients were administered topical dexamethasone 0.1% and ketorolac tromethamine 0.4% for a minimum of 1 month. At least one IVB injection was administered to topical anti-inflammatory treatment-resistant patients. At 1-month post-IVB injection, the patients were re-evaluated and IVB injections were repeated for the patients with an increase in VA (at least 0.1 increase with the Snellen chart) or a decrease in CMT (at least 100 µm with OCT) but who had not reached normal VA and CMT. After one or more IVB injections, patients who had not obtained any significant VA increase or CMT decrease were considered as IVB treatment-resistant patients. Finally, the 16 IVB treatment-resistant patients with PCME underwent IVD (Ozurdex; Allergan, Irvine, CA, USA) implantation. At three months after IVD implantation, the patients were re-evaluated and repeated IVD implantations at three-month intervals were administered for patients who did not have normal VA and CMT. IVD implantation therapy was finalized when normal VA and CMT were obtained or when it was decided that no additional benefit could be gained from further implantations.
Each patient was evaluated regularly with OCT in addition to monthly clinical examinations. The morphology of macular changes such as cystic macular edema or diffuse macular thickening was evaluated using the same OCT device and the same observer. CMT was measured in the same area each time with the aid of an eye tracker system and differences in CMT at the same point of the fovea were calculated with the progression mode of the OCT devices in each evaluation period. An example of the progression in CMT examined by OCT is shown in Fig. 1.
Statistical analyses were performed using the IBM SPSS Statistics ver. 24.0 (IBM Corp., Armonk, NY, USA). The results are presented as mean ± standard deviation. The conformity of numerical data to a normal distribution was evaluated using the Kolmogorov-Smirnov test and the numerical data did not fit a normal distribution, thus the Wilcoxon test was used for statistical analysis of independent variables. A value of p < 0.05 was considered statistically significant.
The female : male ratio was 7 : 9 in this study. Patient mean age was 66.2 ± 3.9 years (range, 57 to 73 years). Four patients had type 2 diabetes mellitus without diabetic retinopathy and were using insulin. Five patients had systemic hypertension and were taking oral anti-hypertensive medication.
The mean duration between cataract surgery and the first IVB injection was 6.3 ± 2.1 months (3 to 11 months). The mean number of IVB injections was 2.19 ± 1.43 (1 to 5). The mean interval between the last IVB injection and the first IVD implantation was 3.6 ± 1.1 months (1 to 6 months).
The mean number of IVD implantations was 1.44 ± 0.89 (1 to 4). In patients who underwent multiple IVD implantations, the mean interval between two IVD implantations was 4.4 ± 1.5 months (3 to 6 months). The mean follow-up time after the first IVD implantation was 7.4 ± 4.6 months (6 to 24 months). All patients who needed repeat IVD implantations had complicated cataract surgery with sulcus IOL implantation. All of these patients also needed more IVB injections compared with patients who underwent uneventful surgery. One of the referred patients had three IVD implantations and required keratoplasty for pseudophakic bullous keratopathy. One patient who had four IVD implantations had vitreous in the anterior chamber due to complicated cataract surgery and this was treated with anterior vitrectomy and anterior chamber restoration after an unsuccessful attempt of Nd:YAG laser vitreolysis. No vitreomacular interface abnormalities were seen in the follow-up period and no patients required vitreoretinal surgery. The treatment was finalized early after the 3rd IVD implantation in one patient (serial number 8) due to permanent low VA and an outer nuclear layer defect. The clinical features, number of intravitreal injections or implantations, pre-IVD implantation and final CMTs, and patient follow-up periods are summarized in Table 1.
Before IVD implantation, the mean VA and CMT were 0.69 ± 0.19 logarithm of the minimum angle of resolution (logMAR) (1.50 to 0.10 logMAR) and 476.13 ± 135.13 mm (314 to 750 mm), respectively. At three months after the first IVD implantation, mean VA and CMT were 0.26 ± 0.07 logMAR (1.00 to 0.00 logMAR) and 294.06 ± 15.26 mm (222 to 480 mm), respectively. The differences between the values before and at 3 months after the IVD implantation values were statistically significant (p < 0.001 for both). The time-based changes in VA and CMT with IVD implantation are summarized in Table 2. At the end of the follow-up period, the mean VA and CMT values in the whole group were 0.19 ± 0.05 logMAR (0.70 to 0.00 logMAR) and 268.38 ± 31.35 mm (217 to 351 mm), respectively. The mean CMT decrement in this series was 207.81 ± 151.13 mm (9 to 530 mm). The CMT values before IVD implantation and at the end of the follow-up periods are shown in Fig. 2.
While the occurrence of PCME has declined with the use of modern surgical techniques, recently developed surgical materials and IOL design, PCME can still occur even following uneventful cataract surgery [15]. Furthermore, the incidence of clinically significant PCME remains around 0.6% to 3.6%, while higher rates have been determined with OCT evidence of CME [51016].
Although the pathogenesis of PCME has been reported to be multifactorial, the activation of inflammatory pathways seems to play a critical role in its onset and continuation. Surgical mechanical trauma triggers a cascade of inflammatory events and increases the synthesis of inflammatory mediators such as prostaglandins and cytokines. Inflammatory mediators cause the breakdown of the blood-retinal barrier that leads to the accumulation of extracellular intra-retinal fluid, resulting in macular thickening and creating cystic spaces [17]. In the current series, the persistence and re-occurrence of macular edema evaluated on OCT was observed more frequently in patients who had complicated surgery than in subjects with uneventful surgery. The number of IVB injections was higher in eyes that had complicated cataract surgery, and repeated IVD implantation was only performed in eyes that had complicated surgery with sulcus IOL implantation. These results provide indirect evidence that surgical complications are important risk factors for the development of treatment-resistant PCME.
Several treatment methods have been applied for PCME, depending on the etiology. Based on the key role of prostaglandins and leukotriene-mediated inflammation in PCME, conventional treatments for PCME have included steroid and non-steroidal anti-inflammatory drugs [18]. Brynskov et al. [19] reported successful treatment with IVD implantation in a subtenon triamcinolone treatment-resistant PCME case. Dang et al. [20] compared the efficacy of intravitreal triamcinolone injection and IVD implantation. The authors reported that both treatments similarly restored VA and CMT, but IVD implantation showed longer efficacy and was well tolerated. Therefore, IVD implantation was suggested as a new treatment option in PCME cases [20]. Garcia et al. [21] applied IVD implantation in a series of six patients and favorable anatomic outcomes via OCT were confirmed. Kakkassery et al. [22] showed the efficacy of IVD implantation in PCME resistant to both several topical and oral medication combinations, including topical diclofenac, topical and oral prednisolone, and oral acetazolamide. Mayer et al. [23] observed that CMT decreased from 520.8 ± 71.4 to 232.7 ± 26.6 µm after IVD implantation for treatment of PCME in 23 patients after uneventful cataract surgery. The decrease in CMT in the current series (mean values, from 476.13 to 207.81 µm) was similar to those findings. However, the previous study reported nine recurrences with a peak at three months after implantation that required a second IVD implantation [23]. Although only patients with uneventful cataract surgery were evaluated in the previous study, the recurrence rate was higher than that of the current series. This result showed that risk factors other than complicated surgery may also be important for the recurrence of PCME. Dutra Medeiros et al. [24] reported that the mean CMT decreased from 542.22 to 143.89 µm at 6 months after a single IVD implantation. The mean decrement in CMT was lower than the current series (207.81 mm). This result demonstrated that single IVD implantation was not sufficient in treatment-resistant cases and repeated injections were required, as in the current series.
Another hypothesis that could explain the increase in endothelial permeability is the vascular endothelial growth factor-associated breakdown of the blood-retinal barrier [25]. Many ophthalmologists use IVB in treatment-naïve or topical anti-inflammatory treatment-resistant cases of PCME. Arevalo et al. [26] used the IVB treatment option in 36 eyes with PCME. After a 12-month follow-up period, a significant improvement was obtained on OCT with a mean of 2.7 IVB injections [24]. Similar findings have been reported by other authors [2728]. In contrast, Spitzer et al. [29] showed that a 1.25 mg IVB injection caused a slight decrease in CMT in a series of 16 eyes with refractory PCME. In our clinical practice, we observed that PCME can be treated with IVB, although there is no single treatment regimen that is suitable for every patient. In this study, we observed that some patients who received a mean of 2.19 repeat IVB injections, similar to the Arevalo et al. series [26], still had persistent macular edema even without any significant ocular and systemic risk factors other than complicated surgery in some. Therefore, only cases resistant to both topical and IVB treatment were evaluated to investigate a better option for these patients.
The main limitation of this study was the lack of a control group for comparison with the IVD-treated group. This approach could clearly show the efficacy of IVD therapy on patients with IVB treatment-resistant PCME. Furthermore, the mean follow-up time after implantation of IVD was 7.4 months, which may be considered a short period. This study also only included 16 patients and this small number restricts results generalization. The current study sample was not fully homogeneous because it included patients with complicated and uncomplicated surgeries. In addition, the patients had not received the same types of treatments before IVD implantation. The retrospective nature of the study is another important limitation.
To the best of our knowledge, this is the first report to show the efficacy of IVD implants even after repeated IVB injections in treatment-resistant PCME. Although there is no widely accepted treatment algorithm for recalcitrant PCME cases, the current findings suggest that prolonged activity of IVD implantation is both a safe and effective method for the resolution of macular edema resistant to other possible treatments after both uneventful and complicated cataract surgery. Further studies with a large number of patients are needed for the development of new treatment algorithms in patients with recalcitrant macular edema resulting from Irvine-Gass syndrome.
Figures and Tables
Fig. 1
An example of the progression in central macular thickness examined by optical coherence tomography (OCT). ILM = internal limiting membrane; BM = Bruch membrane; IR = infrared; HS = high speed; ART = automatic realtime tracking; Q = quality factor.
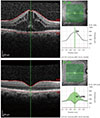
Fig. 2
Central macular thickness values before intravitreal dexamethasone implantation and at the end of the follow-up period.

References
2. Gass JD, Norton EW. Cystoid macular edema and papilledema following cataract extraction: a fluorescein fundoscopic and angiographic study. Arch Ophthalmol. 1966; 76:646–661.
3. Schubert HD. Cystoid macular edema: the apparent role of mechanical factors. Prog Clin Biol Res. 1989; 312:277–291.
4. Ursell PG, Spalton DJ, Whitcup SM, Nussenblatt RB. Cystoid macular edema after phacoemulsification: relationship to blood-aqueous barrier damage and visual acuity. J Cataract Refract Surg. 1999; 25:1492–1497.

5. Henderson BA, Kim JY, Ament CS, et al. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg. 2007; 33:1550–1558.
6. Eriksson U, Alm A, Bjarnhall G, et al. Macular edema and visual outcome following cataract surgery in patients with diabetic retinopathy and controls. Graefes Arch Clin Exp Ophthalmol. 2011; 249:349–359.

7. Chu CJ, Johnston RL, Buscombe C, et al. Risk factors and incidence of macular edema after cataract surgery: a database study of 81984 eyes. Ophthalmology. 2016; 123:316–323.
8. Sacconi R, Corbelli E, Carnevali A, et al. Optical coherence tomography angiography in pseudophakic cystoid macular oedema compared to diabetic macular oedema: qualitative and quantitative evaluation of retinal vasculature. Br J Ophthalmol. 2018; 102:1684–1690.

9. Benitah NR, Arroyo JG. Pseudophakic cystoid macular edema. Int Ophthalmol Clin. 2010; 50:139–153.

10. Vukicevic M, Gin T, Al-Qureshi S. Prevalence of optical coherence tomography-diagnosed postoperative cystoid macular oedema in patients following uncomplicated phaco-emulsification cataract surgery. Clin Exp Ophthalmol. 2012; 40:282–287.

11. Altintas AG, Coban P, Arifoglu HB, et al. Comparison of phaco parameters effect on macular thickness changes after uneventful phacosurgery in diabetic and non-diabetic patients. Int Eye Sci. 2016; 16:201–206.
12. Lin CJ, Tsai YY. Use of aflibercept for the management of refractory pseudophakic macular edema in Irvine-Gass syndrome and literature review. Retin Cases Brief Rep. 2018; 12:59–62.

13. Kiernan DF, Hariprasad SM. Controversies in the management of Irvine-Gass syndrome. Ophthalmic Surg Lasers Imaging Retina. 2013; 44:522–527.

14. Randazzo A, Vinciguerra P. Chronic macular edema medical treatment in Irvine-Gass syndrome: case report. Eur J Ophthalmol. 2010; 20:462–465.

15. Ilhan C. Current developments in monofocal intraocular lens technology. Int J Ophthalmic Res. 2017; 3:239–242.
16. Lobo CL, Faria PM, Soares MA, et al. Macular alterations after small-incision cataract surgery. J Cataract Refract Surg. 2004; 30:752–760.

17. Hudes GR, Li WY, Rockey JH, White P. Prostacyclin is the major prostaglandin synthesized by bovine retinal capillary pericytes in culture. Invest Ophthalmol Vis Sci. 1988; 29:1511–1516.
18. Shelsta HN, Jampol LM. Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update. Retina. 2011; 31:4–12.
19. Brynskov T, Laugesen CS, Halborg J, et al. Longstanding refractory pseudophakic cystoid macular edema resolved using intravitreal 0.7 mg dexamethasone implants. Clin Ophthalmol. 2013; 7:1171–1174.

20. Dang Y, Mu Y, Li L, et al. Comparison of dexamethasone intravitreal implant and intravitreal triamcinolone acetonide for the treatment of pseudophakic cystoid macular edema in diabetic patients. Drug Des Devel Ther. 2014; 8:1441–1449.

21. Garcia JM, Isaac DL, Avila MP. Dexamethasone 0.7 mg implants in the management of pseudophakic cystoid macular edema. Arq Bras Oftalmol. 2016; 79:113–115.

22. Kakkassery V, Schultz T, Wunderlich MI, et al. Evaluation of predictive factors for successful intravitreal dexamethasone in pseudophakic cystoid macular edema. J Ophthalmol. 2017; 2017:4625730.

23. Mayer WJ, Kurz S, Wolf A, et al. Dexamethasone implant as an effective treatment option for macular edema due to Irvine-Gass syndrome. J Cataract Refract Surg. 2015; 41:1954–1961.

24. Dutra Medeiros M, Navarro R, Garcia-Arumi J, et al. Dexamethasone intravitreal implant for treatment of patients with recalcitrant macular edema resulting from Irvine-Gass syndrome. Invest Ophthalmol Vis Sci. 2013; 54:3320–3324.

25. Tolentino MJ, McLeod DS, Taomoto M, et al. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol. 2002; 133:373–385.

26. Arevalo JF, Maia M, Garcia-Amaris RA, et al. Intravitreal bevacizumab for refractory pseudophakic cystoid macular edema: the Pan-American Collaborative Retina Study Group results. Ophthalmology. 2009; 116:1481–1487.
27. Mason JO 3rd, Albert MA Jr, Vail R. Intravitreal bevacizumab (Avastin) for refractory pseudophakic cystoid macular edema. Retina. 2006; 26:356–357.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download