This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
To describe the clinical characteristics and course of optic neuritis (ON) and its association with neuromyelitis optica spectrum disorder (NMOSD) and multiple sclerosis (MS) in Korea.
Methods
In this retrospective case series, 125 eyes of 91 Korean patients with ON were included. The medical documents of adult patients with ON were retrospectively reviewed. Patients were assigned into idiopathic ON, NMOSD, and MS groups according to the presence of an association with NMOSD or MS for subgroup analysis. Clinical characteristics, disease course, and visual and systemic prognosis were analyzed.
Results
During the mean follow-up of 3.7 years, 73 patients were diagnosed as idiopathic ON, 14 patients were diagnosed as NMOSD, and four patients developed definite MS. At the final visit, there were 13 (13%) eyes out of 100 eyes with idiopathic ON, nine (43%) eyes out of 21 eyes with NMOSD, and one (25%) eye out of four eyes with MS had a severe visual loss of 20 / 200 or less. The mean Expanded Disability Status Scale was 3.1 ± 1.5 in NMOSD and 1.8 ± 1.5 in the MS group at the final visit. In the NMOSD group, 50% of patients showed severe visual loss in at least one eye or were unable to ambulate without assistance at the final visit (5.3 ± 4.4 years after the initial episode of ON).
Conclusions
Fourteen percent of patients showed positive results for NMO-immunoglobulin G test and 50% of patients with NMOSD showed a severe visual loss in at least one eye or were unable to ambulate without assistance. The proportion of MS was relatively low in Korean ON patients.
Keywords: Korea, Multiple sclerosis, Neuromyelitis optica, Optic neuritis
Optic neuritis (ON) is one of the common optic neuropathies in young adults, with an annual incidence of 5.1 / 100,000 in industrialized countries [
1]. It is also a common manifestation of multiple sclerosis (MS). Various reports have shown differences in clinical expression of ON in patients of Western or Asian countries [
234567]. In recent decades, neuromyelitis optica (NMO), a demyelinating neurologic disorder, has been found to be strongly associated with ON, especially in Asians. It is a severe idiopathic inflammatory disease of the central nervous system that predominantly affects optic nerves and spinal cord [
8]. Many studies have shown that NMO is more prevalent in Blacks, Asians, and Indians compared to Whites [
910111213]. Because of its poor prognosis, early and aggressive treatment is important for patients with NMO. However, there has been a limited number of studies regarding the proportion of NMO and its prognosis in Asian patients with ON. In addition, serologic tests for NMO are largely performed according to the discretion of each clinician. Clinical studies on patients who were diagnosed as NMO through serologic tests were largely limited in Korea. The objective of this study was to characterize the clinical presentation of ON in Korean patients and its association with NMO and MS over 10 years.
Materials and Methods
A retrospective review was performed for a group of ON patients who were followed-up at the Neuro-ophthalmology Department of Samsung Medical Center from March 2007 to August 2016 according to the tenets of the Declaration of Helsinki. All patients who were initially referred from the Neurology Department were not included in this review to avoid inclusion of patients who were already suspected of having a neurologic disorder, such as NMO spectrum disorder (NMOSD) or MS, at initial presentation. The study was approved by the institutional review board of Samsung Medical Center (2018-07-144). Informed consent was not obtained in this study. Identifying information about participants was not presented in this study.
All patients enrolled had experienced a clinical episode of ON. A history of acute ON episodes was confirmed by medical chart review. Diagnosis of an ON episode was based on clinical symptoms such as gradual visual loss over several days with or without pain on eye movement, documented findings of decreased visual acuity, visual field defect, loss of color vision, relative afferent pupillary defect in case of unilateral involvement, and compatible fundus examination with or without signs of abnormal optic nerve enhancement on magnetic resonance imaging (MRI). Patients with any of the following conditions were excluded: age of less than 20 years, age of 70 years or more, history of any form of neurological impairment or previous diagnosis of neurologic disorder such as NMOSD or MS prior to the first ON episode, any history of systemic vasculitis, malignancy, or other ocular pathologies that could affect visual function including retinal disease and optic neuropathies other than ON such as glaucoma.
Patients with ON were assigned to the idiopathic ON, NMOSD, or MS group according to the presence of an association with NMOSD or MS for subgroup analysis. NMOSD was defined as suggested by Wingerchuk et al. [
14]. The diagnosis of MS was based on the McDonald criteria [
15].
All patients underwent a full ophthalmologic assessment including visual acuity test, color vision test with Ishihara charts, slit lamp biomicroscopy, fundus examination, and routine laboratory tests including complete blood cell count, electrolytes, chemistry profiles, erythrocyte sedimentation rate, C-reactive protein, serologic tests for human immunodeficiency virus and syphilis, and fluorescent antinuclear antibody. The visual field was tested using a Humphrey Field Analyzer with a 30-2 SITA-standard protocol. The test for NMO-immunoglobulin G (IgG) was performed either immediately after serum sampling or after storage at −70℃. NMO-IgG was measured using a commercially available cell-based immunofluorescence assay kit from Euroimmun AG (Lubeck, Germany) according to the manufacturer's instructions [
16]. Brain and orbit MRI of participants were reviewed. Whether the protocol used for image acquisition and image quality was appropriate to analyze optic nerves was determined for every image by one of our authors (KAP).
If the presentation of ON recurred < 30 days from the first attack, it was considered to be a relapse. If it occurred ≥30 days later, it was considered a new attack or a recurrence as defined by McDonald et al. [
17]. The disease severity in patients with NMOSD or MS was assessed via the Expanded Disability Status Scale (EDSS) score [
18].
Statistical analyses were performed using SAS ver. 9.4 (SAS Institute, Cary, NC, USA). To compare baseline characteristics and visual outcomes between idiopathic ON, NMOSD, and MS groups, a Generalized Estimating Equation with an exchangeable working correlation matrix was used for repeated measures from the same subject. To assess factors affecting severe visual loss, univariate and multivariate analyses were performed.
Results
In this study, 125 eyes of 91 patients were included. The demographics and clinical characteristics of the patients were compared in the present study and with other Asian studies and the Optic Neuritis Treatment Trial (ONTT) (
Table 1) [
246718]. Their mean age was 43 ± 13 years (range, 20 to 69 years). Fifty-seven (63%) ON patients were female. Ocular pain was noted in 61 (56%) eyes. Disc swelling was present in 60 (53%) eyes. The mean follow-up duration was 3.7 ± 3.3 years (range, 0.5 to 16 years). Twenty-seven (30%) patients had ON in both eyes simultaneously or sequentially during the follow-up period and 19 (21%) patients presented with bilateral and simultaneous involvement at initial presentation. The initial visual acuity ranged from 20 / 20 to no light perception. Sixty-four (51%) eyes presented with an initial visual acuity of 20 / 200 or worse.
Of 91 patients, 13 (14%) patients had positive results in the NMO-IgG test. Among the 49 patients who underwent an NMO-IgG test, 13 (27%) were positive. Of the 42 patients who visited after 2011, when we first started to check NMO-IgG routinely in ON patients, seven (17%) patients showed positive results, however, seven of the patients did not undergo an NMO-IgG test. Among the seven patients who did not undergo an NMO-IgG test, six patients recovered with a normal vision of 20 / 20 and normal visual field without recurrence. Only one patient with unilateral involvement had no light perception vision during the 6 years of follow-up without any improvement. Among 35 patients who underwent an NMO-IgG test during the initial visit after 2011, the test positivity was 20% (7 / 35) (
Fig. 1).
Images of brain MRI were available for 85 patients. The images revealed that periventricular lesion (PVL) was present in 11 (13%) patients at the initial presentation. Regarding optic nerve enhancement, 92 eyes were evaluated with orbit MRI within 2 weeks of onset using a contrast enhancement and fat suppression technique with a magnified view of the optic nerve. Of these 92 eyes, 68 (74%) showed optic nerve enhancement.
During the follow-up, 73 (80%) patients were diagnosed as idiopathic ON and 14 (15%) were diagnosed as NMOSD. Four (4%) developed definite MS based on clinical grounds. Clinical characteristics in patients according to subgroups are shown in
Table 2. There were significant differences in initial visual acuity and the presence of PVL (
p = 0.008 and
p = 0.023, respectively) according to ON subgroups. Initial visual acuity was the worst in patients with NMOSD. The number of eyes with an initial visual acuity of 20 / 200 or worse was 45 (46%) out of 97 with idiopathic ON, 16 (76%) out of 21 ON patients with NMOSD, and four (100%) out of four ON patients with MS. The presence of PVL was found more often in MS patients (75%).
All but two patients received intravenous methylprednisolone sodium succinate (250 mg four times daily for 3 to 5 days followed by oral prednisolone [1 mg/kg daily] for 11 days). One patient did not receive any treatment because disease severity was mild, and the other patient was pregnant. If the NMO-IgG test was positive, long-term immunosuppressive treatment was initiated after tapering oral prednisolone slower than in the above-mentioned regimen at the Neurology Department. Patients who developed clinically definite MS during follow-up were administered disease modifying drugs such as mycophenolate mofetil (Myrept tab, Chong Kun Dang, Seoul, Korea), interferon β-1a (Rebif, Merck Sharp & Dohme, Whitehouse Station, NJ, USA), interferon β-1b (Betaferon, Bayer AG, Leverkusen, Germany), teriflunomide (Aubagio, Genzyme, Cambridge, MA, USA), glatiramer acetate (Copaxone, Handok, Seoul, Korea) and natalizumab (Tysabri, Eisai, Tokyo, Japan).
Mean follow-up duration was 3.7 ± 3.3 years (range, 0.5 to 16.0 years). Overall seven relapses occurred in our subjects after intravenous methylprednisolone treatment. All of them had idiopathic ON. Ninety-one (73%) eyes achieved visual acuity of 20 / 40 or better. Twenty-four (19%) eyes attained 20 / 200 or less vision. The clinical outcomes of each ON subgroup are shown in
Table 3. There were no significant differences in the final logarithm of the minimum angle of resolution of visual acuity among the ON subgroups. There was a marginally significant difference in the proportion of severe visual loss with a visual acuity of 20 / 200 or less at the final visit among ON subgroups (
p = 0.052). Fourteen (14%) eyes in patients with idiopathic ON, nine (43%) eyes in patients with NMOSD, and one (25%) eye in patients with MS had severe final visual loss. Factors affecting severe visual loss at the final visit were analyzed (
Table 4). Significant predictors of final severe visual loss included initial visual acuity, initial color vision, and initial mean deviation on visual field test (
p < 0.001,
p = 0.010, and
p = 0.007, respectively). Variables with a
p-value of less than 0.1 were included in the multivariate analysis. Initial color vision and initial visual field loss were not included in the final multivariate analysis because of their close relationship with visual acuity. Only the initial visual acuity was found to be a significant factor affecting severe visual loss at the final visit based on multivariate analysis (
p < 0.001).
Among the four patients with MS, three had PVL at the initial presentation. One patient who had no PVL developed neurologic deficit and PVL on MRI and was diagnosed as MS later. Among 14 patients with NMOSD, one patient had multiple PVL at the initial presentation.
Recurrence was noted in 31 (34%) patients. The mean time interval from initial presentation to recurrence was 3.3 ± 6.3 years. Twenty (22%) patients experienced recurrence of ON in either eye or both eyes within 1 year, three (3%) patients had a recurrence at 1 to 3 years after the onset of ON, four (4%) patients had recurrence at 10 years to 24 years after the onset of ON. In one (1%) patient, the exact duration between recurrence and the onset of ON was unclear. Among subgroups, the recurrence rate was 32% in patients with idiopathic ON and 57% in ON patients with NMOSD. There was no recurrence among four patients with MS during the follow-up period. The time interval from the first episode to the first recurrence was 3.3 ± 6.3 years in patients with idiopathic ON. It was 3.7 ± 6.6 months in ON patients with NMOSD.
Six (43%) of 14 ON patients with NMOSD experienced one or more attack of longitudinally extensive transverse myelitis at various degrees within 7 ± 8 months (range, 0 to 24 months) after the first ON episode. The mean EDSS in ON patients with NMOSD was 3.1 ± 1.5 (range, 0.0 to 7.0) at the final visit (5.3 ± 4.4 years after the initial onset of ON). One patient had a disability affecting ambulation at the final visit. The patient used a wheelchair unaided (EDSS 7.0). Two (14%) patients had permanent legal blindness in both eyes and 3 (21%) patients had permanent legal blindness in one eye. In total, seven (50%) patients with NMOSD showed severe visual loss in at least one eye or were unable to ambulate without assistance at the final visit. Three (75%) of four ON patients with MS experienced various degrees of decrease in sensory or pyramidal functions within 11 ± 15 months (range, 1 to 29 months) after the initial onset of ON. The mean EDSS in ON patients with MS was 1.8 ± 1.5 (range, 0.0 to 3.0) at the final visit (4.9 ± 3.8 years after the initial onset of ON). One patient showed permanent blindness in one eye at the final visit.
Discussion
Clinical characteristics of patients in this study were similar to those of previous studies in other Asian countries, including a lower percentage of the female gender, lower percentage of patients with pain, higher percentage of patients with severe initial visual loss, and lower percentage of patients with PVL and MS at presentation compared to data in the ONTT [
23456719].
In this study, retrobulbar optic nerve enhancement was noted in 74% of eyes, which was greater than that (33%) reported in a study by Wang et al. [
4]. The difference could be partly due to differences in the characteristics of participants. It might also be related to the location of ON [
20].
In the report by Wang et al. [
4], disc swelling, which suggested anterior ON, was present in 65% of patients. In the present study, disc swelling was noted in 53% of patients. This difference in the presence of disc swelling could be associated with the different age distribution of the participants between the two studies. Some studies conducted on Asians have analyzed the results of pediatric and adult ON patients together. Pediatric ON patients tend to have more anterior ON than adult ON patients [
21].
During the follow-up in this study, only 4% of ON patients developed definite MS on clinical grounds, consistent with other Asian results showing a relatively low percentage of MS in ON patients [
23456]. The proportion of patients with NMO-IgG antibody was 14%. The proportion of NMOSD was much higher than that of MS in the participants of this study. Although no specific study has presented the proportion of NMOSD among Asian ON patients, many studies have shown that NMO is more prevalent in Black, Asian, and Indian populations compared to Whites [
910111213]. The reason for this difference in etiology might be genetic and environmental influences [
7]. With regards to the poor prognosis and need of long-term immunosuppression in NMO, the high proportion of NMOSD in patients with ON in our study population was of concern. It is noteworthy that the proportion was among patients whose first presentation was ON. It is also remarkable that 21% of NMOSD patients did not show bilateral presentation or severe visual loss at the initial presentation. It is known that early diagnosis and intensive treatments are necessary for patients with NMO. Our data suggest that it is worthwhile performing an NMO-IgG test for every patient at their first attack of ON to allow the initiation of early treatment.
The proportion of patients with PVL at the initial presentation was 13%, which was similar to that (14%) reported in Japan [
2]. Among our study population, 36% of patients with PVL developed MS or NMOSD during the follow-up period. Although the proportion of patients with PVL on MRI at the initial presentation was relatively low in our study population, the presence of PVL in Asian patients also could be regarded as a warning sign for possible association with other neurologic disorders such as MS and NMOSD.
Recurrence was noted in 34% of ON patients in this study. Most cases that (65%) recurred showed recurrence within 1 year. However, recurrence was noted even 24 years after the first episode of ON in one case. The recurrence rate was 32% in patients with idiopathic ON and 57% in ON patients with NMOSD. Probably due to the small number included, there was no recurrent case in the MS group. In our study, 26% of patients with recurrent ON developed NMOSD during the follow-up period. In recurrent cases, an NMO-IgG test needs to be performed based on these results.
According to the ONTT data [
1922], the prognosis of ON is generally good, with 95% of patients having visual recovery ≥20 / 40. In this study, 73% of eyes achieved 20 / 40 visual acuity or better and 19% attained 20 / 200 or less vision. The difference in results could be due to different proportions of Asian patients with ON associated with NMOSD. In this study, 14% of patients in the idiopathic ON group and 43% of patients in the NMOSD group had a severe visual loss at the final follow-up. In multivariate analysis, the initial visual acuity was found to be a significant factor affecting severe visual loss at the final visit. This result was consistent with previous studies [
2324].
After the first ON episode, 43% of ON patients with NMOSD experienced one or more attacks of longitudinally extensive transverse myelitis at various degrees within 0 to 24 months. The mean EDSS in ON patients with NMOSD was 3.1 at the final visit. In this study, 50% of patients showed a severe visual loss in at least one eye or unable to ambulate without assistance within 5.3 ± 4.4 years after the initial onset of ON. With regards to MS in this study, because there were only 4 patients who developed definite MS, caution should be taken when generalizing the characteristics of these patients to others. There were 3 (75%) patients with MS who showed various degrees of decreased sensory or pyramidal functions other than visual symptoms, although the severity was relatively mild (EDSS range, 0.0 to 3.0).
This study has several limitations. First, this study was retrospective in nature. There were also significant variabilities in the length of follow-up for different patients. Second, all patients with NMOSD and MS received some form of long-term immunosuppressive or immunomodulatory treatment. Therefore, the results of this study could not reflect the natural history of these patients after the ON event. Third, due to the relative rarity of MS in patients whose initial presentation was ON in this study, the analysis of this subgroup was largely limited because of the small number of subjects. Fourth, there might be other disorders related to ON that were not diagnosed. For example, we only performed a serologic test for NMO without assessing myelin oligodendrocyte glycoprotein [
2526]. Finally, all study participants were Asians. Therefore, we could not compare the difference between races directly in this study. Because all data were obtained from one ethnicity, the direct application of these data to other races might be dangerous.
In conclusion, we analyzed the characteristics of ON in Korean patients, including test positivity for NMO-IgG and visual and systemic prognosis in these patients according to the presence of an association with NMOSD or MS. In our study, 14% of patients showed positive results in an NMO-IgG test and 50% of patients with NMOSD showed severe visual loss in at least one eye or were unable to ambulate without assistance at the final visit within 5.3 years of the initial onset of ON. Due to the poor prognosis and debilitating course of ON, an NMO-IgG test needs to be performed for every ON patient at the first attack to allow the early initiation of treatment in Asian patients.
Figures and Tables
Fig. 1
Neuromyelitis optica-immunoglobulin G (NMO-IgG) test positivity before and after 2011. Before 2011 when NMO-IgG was not checked routinely in optic neuritis patients, 13 of 91 patients (14%) patients had positive results for the NMO-IgG test. Among 49 patients who underwent the NMO-IgG test, 13 (27%) were positive. After 2011 when we started to check NMO-IgG routinely in optic neuritis patients, 7 (17%) patients showed positive results. However, of 42 patients who visited after 2011, seven patients did not undergo the NMO-IgG test. Among 35 patients who underwent the NMO-IgG test, seven (20%) patients had positive results for the NMO-IgG test.

Table 1
Comparison of patient demographics and clinical characteristics of the present study, other Asian studies, and the ONTT

Table 2
Comparison of patient demographics and clinical characteristics of ON patients according to the presence of an association with NMOSD or MS
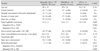
Table 3
Comparison of clinical outcomes of ON patients according to the presence of an association with NMOSD or MS

Table 4
Factors affecting severe visual loss at the final visit in patients with ON










 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download