Abstract
Purpose
To evaluate the efficacy of switching to aflibercept in diabetic macular edema (DME) with suboptimal response to previous anti-vascular endothelial growth factor (anti-VEGF) injections.
Methods
A prospective interventional case series study recruited patients from a single center diagnosed with DME with suboptimal response to anti-VEGF injections. Three consecutive monthly injections of aflibercept were performed. The primary outcome measure was mean change in visual acuity after switching to aflibercept.
Results
Forty-two patients (42 eyes) were included. Baseline logarithm of the minimum angle of resolution (logMAR) visual acuity was 0.87 ± 0.23 and improved significantly to 0.62 ± 0.29, 0.56 ± 0.34, and 0.46 ± 0.35 at 1, 2, and 3 months, respectively, after the first injection. Mean baseline retinal thickness was 451.57 ± 107.09 µm and decreased significantly at 1, 2, and 3 months after switching to aflibercept (346.52 ± 79.03, 328.24 ± 81.98, and 313.71 ± 85.79 µm, respectively). Both visual improvement and mean change in retinal thickness were significant in patients with pre-aflibercept best-corrected visual acuity less than 1.0 logMAR but were not significant in patients with best-corrected visual acuity more than 1.0 logMAR.
Diabetic retinopathy is the leading cause of visual impairment i n the working-age demographic i n developed countries [1]. This problem is likely to increase in the future due to a growing diabetic population, especially in the Middle East [2]. The Early Treatment Diabetic Retinopathy Study was a milestone study that defined laser photocoagulation as the benchmark treatment for preventing visual loss from diabetic macular edema (DME) [3].
Several studies have demonstrated the safety and efficacy of different anti-vascular endothelial growth factor (anti-VEGF) drugs, namely ranibizumab [4] and bevacizumab [5], in the management of DME. Aflibercept gained US Food and Drug Administration approval to treat DME after the phase 3 trials VIVID and VISTA and provided significant visual and morphological improvement in patients suffering from DME [67]. In light of protocol T of the DRCR.net [8], many retinal specialists are now considering the use of aflibercept in DME cases, especially those with poor presenting vision. A growing body of research is investigating different treatment protocols for management of resistant DME. Switching from bevacizumab or ranibizumab to aflibercept is one promising strategy for addressing this challenging situation [91011]. This positive effect could be explained by the different molecular structure [12], greater binding affinity [13], and/or longer intra-vitreal bio-availability of aflibercept [14].
This study aims to evaluate the short-term visual and retinal morphological changes and safety of switching to af libercept injections in DME refractory to other anti-VEGF drugs.
A prospective interventional case series study was conducted from October 2015 to November 2016 after approval of the Ethical Committee of the Faculty of Medicine, Assiut University, Egypt (264-15). All procedures were carried out under the tenets of the Helsinki Declaration. Written consent was provided by all participants after discussing the procedure, alternative treatment plans, follow-up schedules, and possible benefits and risks.
Patients suffering from previously diagnosed macular edema secondary to type 1 or 2 diabetes mellitus were included. For transition to aflibercept 2 mg/0.05 mL, a diagnosis of resistant DME was required. Patients fulfilling one or more of the following criteria were considered to have resistant DME after at least 3 consecutive monthly bevacizumab 1.25 mg or ranibizumab 0.5 mg injections in the previous 6 months: central macular thickness greater than 300 µm by spectral-domain optical coherence tomography (SD-OCT), reduction of retinal thickness by less than 10% of baseline retinal thickness, or suboptimal visual improvement (failure to gain at least 3 lines on the Snellen chart). Exclusion criteria were unwillingness to participate, significant cataract or corneal opacity, DME associated with proliferative diabetic retinopathy, history of laser treatment in the previous 6 months (either focal or macular grid laser photocoagulation), history of steroid injection in the previous 6 months (peri-ocular or intra-vitreal injection/implant), co-existing retinal pathology (e.g., retinal vascular occlusion, age-related macular degeneration), history of cataract surgery in the previous 12 months, associated optic nerve disorders (e.g., ischemic optic neuropathy), glycosylated hemoglobin higher than 8% at the time of participation, ischemic heart disease, or previously complicated intra-vitreal injection of anti-VEGF. Participants were excluded from the study if they had fewer than 3 consecutive aflibercept intra-vitreal injections.
All participants underwent thorough ophthalmic and systemic evaluation. Ophthalmic evaluation included detailed history-taking, best-corrected visual acuity (BCVA) by Snellen chart (converted subsequently to equivalent logarithm of the minimum angle of resolution [logMAR] values for statistical analysis), slit-lamp examination, and intraocular pressure (IOP) measurement before papillary dilatation (Goldmann applanation tonometer), followed by dilated fundus examination. Fundus fluorescein angiography (Topcon fundus camera; Topcon, Tokyo, Japan) and SD-OCT (Heidelberg Engineering, Heidelberg, Germany) were performed on every participant at initial evaluation.
In SD-OCT assessment, a 30 degree by 30 degree macular grid scan acquisition was carried out with 64 horizontal lines, spaced 50 µm and averaged to 30 frames. Early Treatment Diabetic Retinopathy Study macular thickness map was then measured automatically with Heidelberg software. Poor OCT images (signal strength value below 10) were excluded. Retinal thickness was measured as the perpendicular line between the internal limiting membrane and Bruch's membrane. Central 1 mm macular thickness was recorded with additional manual adjustment for measurement lines. Analysis and interpretation of OCT images were performed by an experienced ophthalmologist (ZE).
All participants received pre-injection prophylactic topical gatifloxacin 0.3% eye drops and topical anesthetic benoxinate 0.4% eye drops. After digital ocular massage to lower the IOP, the intra-vitreal injections were carried out under strict aseptic conditions in the operating room. The injection was performed in the temporal sclera 3.5 mm from the limbus after sweeping conjunctiva with a 5% Povidone Iodine-soaked sterile sponge. Assessment of pupillary response, detection of hand movement, and fundus examination (if needed) were carried out to exclude possible post-injection IOP spikes. Intra-vitreal injection of aflibercept was performed by a single experienced ophthalmologist (WI).
All patients received topical gatifloxacin 0.3% eye drops post-injection for 5 days. Follow-up visits were scheduled at 1, 2, and 3 months after the initial injection. BCVA, slitlamp examination, IOP measurement, and follow-up SD-OCT were performed at each injection.
DME edema was stratified into spongiform macular edema, cystoid macular edema with or without the presence of subretinal fluid, and/or vitreo-macular interface abnormality (VMI). Subretinal fluid was defined as accumulation of fluid between the neuro-sensory retina and retinal pigment epithelium. VMI abnormality was tagged by the presence of high reflective epi-retinal membrane (ERM), antero-posterior vitreo-macular traction, or thickened posterior hyaloid. Retinal morphological analysis was performed at baseline and at 3 months post-injection by SD-OCT and was interpreted by an experienced ophthalmologist (ZE).
The primary outcome measure was mean change in visual acuity after 3 consecutive monthly injections of aflibercept. Secondary outcome measures were mean change in retinal thickness, correlation between baseline visual acuity/baseline retinal thickness and final 3-month visual acuity/retinal thickness, and retinal architectural changes after switching to aflibercept.
Quantitative data were presented as mean ± standard deviation. For comparison between mean BCVA and CMT change pre- and post-aflibercept injection, we used paired sample t-test. Comparison of means among subgroups was carried out by Mann-Whitney test. We utilized Pearson correlation coefficient for correlation between mean post-injection BCVA and central macular thickness (CMT). Statistical significance was considered significant if p-value < 0.05. Statistical analysis was carried out using IBM SPSS Statistics ver. 20.0 (IBM Corp., Armonk, NY, USA).
An interventional case series study recruited 66 consecutive participants. Fourteen participants were excluded because they had received fewer than 3 consecutive intra-vitreal injections of a single anti-VEGF agent (10 patients) or 3 non-consecutive intra-vitreal injections (4 patients). Ten additional participants were excluded because they received only 2 intra-vitreal aflibercept injections and refused to have the third injection. A total of 42 patients (42 eyes) met the inclusion criteria and were included in the study. Mean age of the included patients was 60.04 ± 6.89 years (range, 49 to 71 years). Twenty-six males and 16 females were included in the study. Table 1 demonstrates patient characteristics and baseline assessment.
Mean pre-aflibercept injection CMT was 451.57 ± 107.09 µm, which decreased significantly at 1, 2, and 3 months after switching to aflibercept (346.52 ± 79.03, 328.24 ± 81.98, and 313.71 ± 85.79, respectively, p < 0.001) (Table 2). The patients were was subdivided into two groups based on baseline CMT: less than or equal to 450 µm and more than 450 µm. At the 3-month follow-up, mean reduction of CMT was 126.55 ± 34.71 and 150.30 ± 118.06 µm, respectively, in the two groups (p = 0.44, Mann-Whitney test).
At baseline, mean logMAR visual acuity was 0.87 ± 0.23, which improved significantly to 0.62 ± 0.29 after a single aflibercept 2.0 injection (p = 0.04). Visual acuity continued to improve significantly after the second (0.56 ± 0.34, p = 0.02) and the third aflibercept injections (0.46 ± 0.35, p = 0.03) (Table 3). Furthermore, mean logMAR visual acuity at 3-month follow-up was 0.25 ± 0.17 (equivalent to 6 / 12 on the Snellen chart) in patients with CMT less than o r e qual to 450 µm (22 e yes) a nd 0.68 ± 0.36 (equivalent to 6 / 30 on the Snellen chart) in patients with CMT more than 450 µm (20 eyes) (p = 0.03, Mann-Whitney test). On the other hand, patients who presented with logMAR visual acuity greater than or equal to 1.0 (14 eyes) had a final logMAR visual acuity of 0.8 ± 0.33 (equivalent to 6 / 38 on the Snellen chart), while patients who presented with logMAR visual acuity less than 1.0 (28 eyes) had a final logMAR visual acuity of 0.28 ± 0.21 (equivalent to 6 / 12 on the Snellen chart).
Both baseline visual acuity and retinal thickness had a strong and significant relationship to final logMAR visual acuity and retinal thickness at 3 months (Pearson correlation coefficient 0.64, p < 0.001 and 0.66, p < 0.001, respectively).
In SD-OCT follow-up scans, most of the eyes that presented with spongiform macular edema, intra-retinal cysts, and subretinal fluid at the time of shifting to aflibercept showed improvement by the third month (Fig. 1A, 1B). Progression of VMI abnormality was not found in any participant, and 1 eye developed ERM by the third month.
No serious systemic adverse events (e.g., cerebro-vascular stroke, myocardial infarction) were recorded during the study. Only four cases of subconjunctival hemorrhage were reported, with no other serious ocular adverse events (e.g., endophthalmitis, vitreous hemorrhage, retinal detachment).
DME is one of the major causes of visual impairment in diabetic patients, especially in the working age group [1]. Despite the evolution of multiple treatment modalities for DME since implementation of the macular laser, it is not uncommon to experience DME that has failed to respond adequately to one of the treatment options [3]. There is ongoing debate about the definition of unsatisfactory treatment in DME. Some authors advocate that unsatisfactory response in DME is diagnosed when reduction of retinal thickness is suboptimal; others define it as inadequate visual improvement, while others may combine several parameters [1115]. Suboptimal response in DME could be attributed to many post ulated mechanisms such as tachyphylaxis [1617] or tolerance (due to receptor dysregulation or neutralizing antibody formation against the anti-VEGF agent) [181920]. Many inf lammatory mediators have been implemented in the development and progression of diabetic retinopathy [21]. High VEGF level in the vitreous of diabetic patients could play a role in the pathogenesis and treatment response of DME [22].
There are many strategies in the management of resistant DME, such as switching to another anti-VEGF [23], switching to sustained-release steroid implants [24], combining treatments [25], or surgical intervention [26]. Continuation of anti-VEGF in the absence of satisfactory response was also suggested based on a proposed category of “late responders” [27]. In these eyes, it takes time and multiple injections to challenge high VEGF levels in the retina and/or vitreous, indicating that continuation of the original anti-VEGF may be the solution. The active aflibercept molecule acts differently than those of bevacizumab and ranibizumab. It is a fusion protein with an intermediate molecular weight of 115 KDa, which is between those of bevacizumab (149 KDa) and ranibizumab (48 KDa). The active aflibercept molecule interacts not only with VEGF, but also with placental-derived growth factor, widening the spectrum of its action [12].
The role of poor glycemic control in development and progression of diabetic retinopathy and DME cannot be overlooked [28]. Therefore, we included patients with glycosylated hemoglobin less than 8% in a trial to eliminate that possible confounding factor. We also included patients who had undergone at least 3 consecutive intravitreal injections of anti-VEGF, as many studies recommend 3 consecutive loading doses.
Our study revealed significant visual improvement after aflibercept injection along with significant reduction in retinal thickness. Most of the visual improvement and retinal thickness reduction was obtained after the first aflibercept injection. This does not support or invalidate certain postulated theories mentioned before, as early response could be attributed to tachyphylaxis to drugs other than aflibercept, new interactions between VEGF and fusion protein (aflibercept), or targeting of multiple inflammatory mediators. Further molecular and clinical studies are needed to justify one theory over the other.
In DRCR.net protocol T, aflibercept was superior to other anti-VEGF in treatment-naive eyes when baseline visual acuity was 6 / 15 or worse, though the difference was not clinically or statistically significant [29]. However, aflibercept did not behave in a similar fashion in previously treated eyes. Despite significant reduction in central retinal thickness, eyes with pre-aflibercept retinal thickness greater than 450 µm did not show much improvement compared to patients who presented with retinal thickness less than 450 µm. Also, patients with visual acuity better than 1.0 logMAR had a better response than patients who presented with vision worse than 1.0 logMAR. This could merely reflect the importance of baseline visual acuity as a significant predictor of final visual outcome. It is noteworthy that the number of injections did not differ between the 2 groups (6.57 vs. 6.21 injections in patients with baseline visual acuity <1.0 and >1.0 logMAR, respectively). As long as similar injections are given alongside a DME treatment plan over months, it is w ise to receive anti-VEGF treatment early and regularly. Early intervention will maintain good vision rather than resorting to need for anti-VEGF treatment later when baseline vision is worse and the expected final visual improvement will be unsatisfactory, both to physician and patient.
Many predictors for DME treatment response have been analyzed in an attempt to identify patients who would achieve satisfactory results and who would not. Many studies have concluded that visual acuity at the time of presentation is an important predictor of final visual outcome [3031]. This hypothesis holds not only in treatment-naive eyes, but also in previously injected eyes. We reported that patients with good pre-aflibercept visual acuity will gain more visual improvement than those with poor pre-aflibercept visual acuity (1.13 to 0.79 logMAR when baseline vision was <1.0 logMAR in comparison with 0.73 to 0.29 logMAR when baseline vision was >1.0 logMAR).
Retinal structural improvement under aflibercept treatment was obvious in the current study but did not necessarily reflect visual gain. In the era of OCT, different retinal structural clues could be linked to resistant DME, such as intra-retinal high reflective foci. Also, other retinal architectural parameters could be associated with suboptimal visual improvement, such as ISOS junction integrity [10], outer retinal layers thickness [32], disorganization of inner retinal layers [33], and inconsistent OCT angiography findings [34]. However, the present study did not analyze the relationships between functional changes and prognostic OCT parameters. There are variable results from different studies about the interactions between anti-VEGF agents and VMIs [3536]. In the current study, ERM developed in one eye with no obvious changes in other eyes with VMI after switching to aflibercept injection. In addition, cases with VMI at baseline evaluation showed a variable response to injection-shift. A larger series of cases is needed to verify the potential effect of VMI on treatment response in resistant cases.
Cumulative results of different studies regarding switching to aflibercept in resistant cases of DME are expected to determine potential benefits of the wider spectrum of action of aflibercept in comparison to other anti-VEGFs. A randomized study with longer follow-up is needed to guarantee the reproducibility of the current study findings.
Suboptimal response to anti-VEGF injection in DME is a challenging situation in diabetic retinopathy management. Switching to aflibercept after previous anti-VEGF injections provided acceptable anatomical and functional improvement and should be considered as a promising strategy for resolving this issue.
Figures and Tables
Fig. 1
(A) Retinal architectural changes between baseline and (B) 3 months after switching to aflibercept injection. Though variable morphological changes were observed, overall anatomical improvement was achieved.
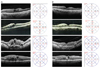
Table 1
Baseline patient characteristics

Values are presented as mean ± standard deviation, number, or number (%).
BCVA = best-corrected visual acuity; logMAR = logarithm of minimal angle of resolution; NPDR = non-proliferative diabetic retinopathy; VEGF = vascular endothelial growth factor; IVTA = intra-vitreal triamcinolone acetonide; TA = triamcinolone acetonide.
Table 2
Retinal thickness changes after shifting to aflibercept from baseline to 3 months after injection

References
1. Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016; 44:260–277.


2. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35:556–564.


3. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985; 103:1796–1806.

4. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized t r ials: R ISE and R IDE. Ophthalmolog y. 2012; 119:789–801.
5. Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012; 130:972–979.


6. Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015; 122:2044–2052.

7. Do DV, Nguyen QD, Boyer D, et al. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012; 119:1658–1665.


8. Diabetic Retinopathy Clinical Research Network. Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015; 372:1193–1203.



9. Rahimy E, Shahlaee A, Khan MA, et al. Conversion to aflibercept after prior anti-VEGF therapy for persistent diabetic macular edema. Am J Ophthalmol. 2016; 164:118–127.


10. Bahrami B, Hong T, Zhu M, et al. Switching therapy from bevacizumab to aflibercept for the management of persistent diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2017; 255:1133–1140.


11. Chen YY, Chang PY, Wang JK. Intravitreal aflibercept for patients with diabetic macular edema refractory to bevacizumab or ranibizumab: analysis of response to aflibercept. Asia Pac J Ophthalmol (Phila). 2017; 6:250–255.


12. Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012; 15:171–185.



13. Moradi A, Sepah YJ, Sadiq MA, et al. Vascular endothelial growth factor trap-eye (Aflibercept) for the management of diabetic macular edema. World J Diabetes. 2013; 4:303–309.



14. Stewart MW, Rosenfeld PJ, Penha FM, et al. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye). Retina. 2012; 32:434–457.


15. Pacella F, Romano MR, Turchetti P, et al. An eighteen-month follow-up study on the effects of Intravitreal Dexamethasone Implant in diabetic macular edema refractory to anti-VEGF therapy. Int J Ophthalmol. 2016; 9:1427–1432.



16. Schaal S, Kaplan HJ, Tezel TH. Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology. 2008; 115:2199–2205.


17. Gokce G, Durukan AH, Koylu MT, Kucukevcilioglu M. Efficacy of aflibercept on exudative age-related macular degeneration in patients exhibiting complete ranibizumab resistance and tachy phylaxis. Arq Bras Of talmol. 2016; 79:384–389.

18. Arjamaa O, Minn H. Resistance, not tachyphylaxis or tolerance. Br J Ophthalmol. 2012; 96:1153–1154.

19. Binder S. Loss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? Br J Ophthalmol. 2012; 96:1–2.


20. Forooghian F, Chew EY, Meyerle CB, et al. Investigation of the role of neutralizing antibodies against bevacizumab as mediators of tachyphylaxis. Acta Ophthalmol. 2011; 89:e206–e207.

21. Praidou A, Androudi S, Brazitikos P, et al. Angiogenic growth factors and their inhibitors in diabetic retinopathy. Curr Diabetes Rev. 2010; 6:304–312.


22. Krizova L, Kalousova M, Kubena AA, et al. Correlation of vitreous vascular endothelial growth factor and uric acid concentration using optical coherence tomography in diabetic macular edema. J Ophthalmol. 2015; 2015:478509.

23. Ferris FL 3rd, Maguire MG, Glassman AR, et al. Evaluating effects of switching anti-vascular endothelial growth factor drugs for age-related macular degeneration and diabetic macular edema. JAMA Ophthalmol. 2016; 12. 22. DOI: 10.1001/jamaophthalmol.2016.4820.

24. Khan Z, Kuriakose RK, Khan M, et al. Efficacy of the intravitreal sustained-release dexamethasone implant for diabetic macular edema refractory to anti-vascular endothelial growth factor therapy: meta-analysis and clinical implications. Ophthalmic Surg Lasers Imaging Retina. 2017; 48:160–166.


25. Maturi RK, Bleau L, Saunders J, et al. A 12-month, single-masked, randomized controlled study of eyes with persistent diabetic macular edema after multiple anti-vegf injections to assess the efficacy of the dexamethasone-delayed delivery system as an adjunct to bevacizumab compared with continued bevacizumab monotherapy. Retina. 2015; 35:1604–1614.


26. Ghassemi F, Bazvand F, Roohipoor R, et al. Outcomes of vitrectomy, membranectomy and internal limiting membrane peeling in patients with refractory diabetic macular edema and non-tractional epiretinal membrane. J Curr Ophthalmol. 2016; 28:199–205.



27. Pieramici DJ, Wang PW, Ding B, Gune S. Visual and anatomic outcomes in patients with diabetic macular edema with limited initial anatomic response to ranibizumab in RIDE and RISE. Ophthalmology. 2016; 123:1345–1350.


28. Prabhu M, Kakhandaki A, Chandra KR, Dinesh MB. A hospital based study regarding awareness of association between glycosylated haemoglobin and severity of diabetic retinopathy in type 2 diabetic individuals. J Clin Diagn Res. 2016; 10:NC01–NC04.

29. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016; 123:1351–1359.


30. Dugel PU, Hillenkamp J, Sivaprasad S, et al. Baseline visual acuity strongly predicts visual acuity gain in patients with diabetic macular edema following anti-vascular endothelial growth factor treatment across trials. Clin Ophthalmol. 2016; 10:1103–1110.



31. Wells JA, Glassman AR, Jampol LM, et al. Association of baseline visual acuity and retinal thickness with 1-year efficacy of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema. JAMA Ophthalmol. 2016; 134:127–134.



32. Eliwa TF, Hussein MA, Zaki MA, Raslan OA. Outer retinal layer thickness as good visual predictor in patients with diabetic macular edema. Retina. 2018; 38:805–811.


33. Sun JK, Radwan SH, Soliman AZ, et al. Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes. 2015; 64:2560–2570.



34. Lee J, Moon BG, Cho AR, Yoon YH. Optical coherence tomography angiography of DME and its association with anti-VEGF treatment response. Ophthalmology. 2016; 123:2368–2375.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download