Abstract
Purpose
To evaluate the efficacy and safety of pterygium excision using a large conjunctival autograft for the treatment of recurrent pterygium.
Methods
The medical records of 120 patients (126 eyes) with recurrent pterygium were reviewed. For each affected eye, pterygium excision with a large conjunctival autograft was performed. The graft was harvested from the superior bulbar area and measured more than 8 × 10 mm in size. Only patients who completed at least six months of follow-up were included. Postoperative clinical outcomes, recurrence rate, and complications were analyzed. Patients with any evidence of recurrence after surgery received a subconjunctival bevacizumab injection.
Results
The average patient age was 56.5 ± 10.2 years, and 45 out of 120 patients were male. The mean study follow-up period was 17.7 ± 17.6 months. Most patients were satisfied with the cosmetic outcome. Postoperative visual acuity improved from 0.69 to 0.75 (p < 0.05). Postoperative refractive astigmatism and corneal astigmatism decreased by 0.55 and 2.73 diopters, respectively (p < 0.05). The postoperative recurrence rate was 4.0%, and the average recurrence period was 7.4 ± 0.6 weeks. A subconjunctival injection of 5 mg bevacizumab was performed in cases of recurrence; no progression of the pterygium was observed following the injection. Postoperative complications included 2 cases of conjunctival graft edema in 2 eyes, 5 donor site scars in 5 eyes, 13 pyogenic granulomas in 13 eyes, and a conjunctival epithelial inclusion cyst in 7 eyes.
Conclusions
Pterygium excision with a large conjunctival autograft for the treatment of recurrent pterygium produced an excellent cosmetic outcome, a low recurrence rate, and minimal complications. A subconjunctival bevacizumab injection given in cases of recurrence following surgery might be effective in preventing progression of the pterygium.
Pterygium is a common conjunctival degenerative change that appears as a wing-shaped growth of fibrovascular tissue on the cornea. Yoon et al. [1] reported that the prevalence of pterygium in Korea was 8.9% in people above 40 years and 16% in people above 60 years. Ultraviolet light and inflammation are presumed to be causative factors in the development of pterygium [23]. No specific treatment is necessary in the case of a small-sized pterygium that causes no irritating symptoms. However, surgical excision is recommended for a pterygium that is cosmetically unacceptable, limits ocular motility, causes a visual disturbance due to its large size, or results in the development of severe astigmatism [45]. There is an increased need to surgically excise recurrent pterygiums due to the presence of more aggressive fibrovascular proliferation and increased adhesion to adjacent tissues [45]. Although there are diverse treatment options available for the management of recurrent pterygium, the recurrence rate is as high as 55% [678910]. Various adjunctive treatment methods, such as amniotic membrane transplantation and administration of an anti-vascular endothelial growth factor (anti-VEGF) drug or mitomycin C, have been used to reduce the recurrence rate after pterygium excision [678910111213141516171819202122232425262728]. However, the reported clinical outcomes of these treatment methods vary in study design, patient characteristics, geographic location, definition of recurrence, and follow-up period [11].
Conjunctival autograft remains the preferred treatment method for recurrent pterygium because it avoids the unacceptable serious complications of single-dose mitomycin C, such as scleral melting and corneal endothelial cell loss [2930313233], and provides a lower recurrence rate and a better cosmetic result than amniotic membrane transplantation [71634]. Although the conjunctival autograft procedure has shown successful results, few studies use this method due to the size of surgical excision required to close the large conjunctival defect [735]. However, Hirst [6] reported that pterygium removal followed by extended conjunctival transplantation for primary pterygium resulted in a near 0% recurrence rate with minimal complications and a good cosmetic appearance.
In this study, we analyzed the clinical outcomes, recurrence rate, and complications following excision of a recurrent pterygium combined with placement of a conjunctival autograft measuring more than 8 × 10 mm. Furthermore, we administered a subconjunctival bevacizumab injection in cases of recurrence following surgery and observed the effects of this medication.
This study was approved by the institutional review board of Cheil Eye Hospital (CEH-2014-6). This study adhered to the tenets of the Declaration of Helsinki. The informed consent was waived. We retrospectively reviewed the medical records of 120 patients diagnosed with recurrent pterygium who underwent surgery performed by the same surgeon (YJP) between September 2007 and August 2014. We assessed their demographic variables, history of recurrence, pterygium size and grade [12], corneal astigmatism, refractive astigmatism, and uncorrected visual acuity. Pterygiums were categorized as grade 1 (atrophic), in which the episcleral vessels under the body of the pterygium were clearly distinguishable; grade 2 (intermediate); or grade 3 (fleshy), where the episcleral vessels were totally obscured. We performed a subgroup analysis according to severity of recurrence. The recurrence group was divided into subgroups according to the Tan classification system, and these subgroups were compared.
The inclusion criteria for participants were as follows: patients (1) who had previously undergone pterygium surgery and had been diagnosed with recurrence; (2) who demonstrated severe ocular motility limitations, visual disturbance, or cosmetic problems; and (3) with a follow-up period longer than six months.
The exclusion criteria were patients (1) who had been diagnosed with or were suspected to have glaucoma in the affected eye; (2) who had not cooperated during the pterygium excision surgery; (3) who had pseudopterygium, a proliferation of fibrovascular conjunctiva secondary to injury; (4) who had a severe ocular surface disease like blepharitis, which is an infection of the ocular surface; or (5) who had been diagnosed with a systemic disease that might be a contraindication for ocular surgery.
Prior to the procedure, written informed consent for the surgical management of a pterygium was obtained from each patient. All surgeries were performed by the same surgeon (YJP). The surgical technique was based on that described by Kim et al. [36] and differed only in regard to the suturing procedure (Fig. 1A, 1B, 1C, 1D, 1E, 1F, 1G, 1H). All patients received topical anesthesia in the form of 0.5% proparacaine hydrochloride (Alcaine; Alcon, Fort Worth, TX, USA). The eye that required surgery was prepared and draped in the usual sterile fashion. After insertion of a lid speculum, the pterygium was injected with 2.0% lidocaine. The head of the pterygium near the limbus was cut using Westcott tenotomy scissors. Then, the scissors were used to dissect the head of the pterygium from the corneal surface in the direction of the central cornea. The pterygium tissue remaining on the corneal and limbal surfaces was smoothed by scraping with a no. 15 Bard-Parker blade. After lifting the free edge of the pterygium, Tenon's capsule tissue was separated from the overlying conjunctiva, and a large amount was excised by cutting both upward and downward toward the conjunctival fornices as well as medially toward (but not reaching) the caruncle. The fibrovascular tissue covering the sclera was meticulously removed using Westcott tenotomy scissors. Minimal wet field cautery was used to control bleeding. Mitomycin C was not applied to the surgical site. After pterygium excision, the eyeball was rotated downward, and limbal-conjunctival tissue was harvested from the superior portion of the same eye. The size of the donor conjunctival tissue was larger than 8 × 10 mm. Blunt dissection of the recipient conjunctiva excluding Tenon's capsule was performed. After fibrin glue (Tissucol duo quick; Baxter AG, Vienna, Austria) was applied to the bare sclera, the donor conjunctival tissue was sutured to the recipient site using several simple interrupted sutures with 10-0 nylon (Ethilon; Johnson & Johnson Medical, Cincinnati, OH, USA) at the tissue margins in order to prevent dehiscence of the donor-recipient junction and subsequent formation of a granuloma. After the conjunctival autograft was secured, temporary amniotic membrane transplantation was performed in all patients to reduce the pain caused by surgical wounds. The human amniotic membrane was removed from its nitrocellulose backing and was placed epithelial side down over the conjunctival autograft site, the defect created in the corneal epithelium by removal of the pterygium head, and the conjunctival donor site. It was secured in place using 10-0 Ethilon at the four corners.
Postoperatively, tobramycin and dexamethasone eye drops (Tobradex; Alcon, Puurs, Belgium) were applied four times a day; of loxacin ointment (Tarivid; Santen, Osaka, Japan) was applied at bedtime for one month to reduce inflammation and pain. On the seventh postoperative day, the amniotic membrane and stitches were removed using a 27-gauge needle and forceps. The patients were evaluated on postoperative days 2, 5, 7, 15, and 30 and then monthly for six months. At that time, r eevaluation was recommended every three months for an additional six months. Thereafter, regular yearly follow-up examinations were encouraged.
During each visit, measurements of visual acuity, intraocular pressure, autorefraction, and autokeratometry were obtained. The surgical wound was carefully evaluated using slit-lamp biomicroscopy for any recurrence of the pterygium or abnormalities in healing. Recurrence of the pterygium was defined as invasion of fibrovascular tissue beyond the corneal limbus. Complications such as graft edema, donor site scarring, pyogenic granuloma, or formation of a conjunctival inclusion cyst were carefully screened for. If there was evidence of clinical recurrence, such as extension of fibrovascular tissue beyond the corneal limbus, 5.0 mg (0.2 mL) subconjunctival bevacizumab (Avastin; Genentech Inc., South San Francisco, CA, USA) was injected biweekly until no further progression was seen.
Data were analyzed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA), and values are reported as mean ± standard deviation. The paired t-test was used to compare changes in uncorrected visual acuity and refractive and corneal astigmatism after treatment. Student's t-test was used to perform subgroup analysis due to severity of recurrence. A p-value less than 0.05 was accepted as statistically significant.
A total of 126 eyes in 120 patients were included in this study. The mean age of the participants was 56.45 ± 10.19 years. The male-to-female ratio was 45 : 75. The average length of the postoperative follow-up period was 17.7 ± 17.6 months. Twenty-two patients had hypertension, and four patients had been diagnosed with diabetes mellitus. Eighteen participants had familial history of pterygium. The number of previous pterygium surgeries was 1.17 ± 0.47. The mean Tan classification was 2.90 ± 0.30. The mean size of the pterygium was 4.77 × 2.90 ± 1.16 × 1.15 mm on the cornea (Table 1). After surgery, the site was re-epithelialized within one to two weeks, and none of the conjunctival grafts became dislodged.
In this study, 3.97% of participants were younger than 40 years of age; 21.43% were aged from 40 to 49 years, 38.89% belonged to the age group of 50 to 59 years, 23.81% were in the age group of 60 to 69, and 11.90% were 70 or older.
Existing cosmetic problems, such as conjunctival injection and fibrovascular tissue covering the cornea, were improved postoperatively (Fig. 2A, 2B). Limitations in ocular motility due to symblepharon or fibrous tissue contraction of the cornea also showed improvement after surgery (Fig. 3A, 3B).
Refractive and corneal astigmatisms had decreased postoperatively from 1.88 ± 2.22 to 1.08 ± 1.05 and from 3.90 ± 3.90 to 1.24 ± 1.09, respectively (p = 0.000). In addition, uncorrected visual acuity improved from 0.69 ± 0.30 to 0.75 ± 0.26 (p = 0.004).
Pterygiums in 26 eyes (20.63%) were categorized as grade 2, while those of 100 eyes (79.37%) were classified as grade 3; none were categorized as grade 1. Preoperative refractive and corneal astigmatisms were significantly greater with grade 3 pterygiums (p = 0.000), but there was no significant difference (p = 0.109) in preoperative uncorrected visual acuity. Postoperative uncorrected visual acuity was significantly improved after surgery for both grade 2 and grade 3 pterygiums (p = 0.035; p = 0.032). There was also significant postoperative improvement in refractive and corneal astigmatisms for grade 3 pterygiums (p = 0.001). The postoperative results were better for grade 2 pterygiums, but the degree of improvement was greater for grade 3 pterygiums (Table 2).
Graft edema that persisted for more than three months after surgery was seen in two eyes (1.5%) (Fig. 4A). However, this condition resolved spontaneously with time. Donor site scarring was observed in five eyes (4.0%) (Fig. 4B). Pyogenic granuloma developed in 13 eyes (10.3%) (Fig. 4C), and conjunctival inclusion cysts were seen in seven eyes (5.6%) (Fig. 4D).
The pterygium recurred in five of the 126 eyes (4.0%): two males and three females. The pterygium reappeared during the seventh postoperative week in two eyes, while it recurred during the eighth week in three eyes. Patients with a recurrence had no hypertension, diabetes mellitus, or familial history of pterygium. The pterygium recurred in one eye (20.00%) classified as grade 2 and in four eyes (80.00%) determined to be grade 3. An injection of 5.0-mg subconjunctival bevacizumab (0.2 ml) was administered in cases that experienced recurrence. The average number of injections in each case of recurrence was 2.4 (range, 1 to 4), and the interval between injections was two weeks. There was no progression of fibrovascular tissue after completion of the subconjunctival bevacizumab injections (Fig. 5A, 5B, 5C). No other complications such as infection, scleral necrosis or thinning, or development of calcific plaques were observed.
According to previous reports, recurrence rates following pterygium excision combined with conjunctival autograft and adjuvant therapies vary according to the method used [67813141516]. Rates of recurrent pterygium ranged from 12.5% to 33% [910171837]. In contrast, few studies have evaluated the effects of one specific treatment on the management of recurrent pterygium in a large number of patients. Conjunctival autograft for recurrent pterygium remains the preferred method. Successful results have been reported with conjunctival autografts [71634]. In general, this type of autograft is not feasible for large defects [735]. However, Hirst [6] reported that aggressive excision of the pterygium accompanied by placement of a large conjunctival autograft resulted in a near 0% recurrence rate with minimal complications and a good cosmetic appearance. Therefore, in this study, we focused on extensive removal of conjunctival fibrovascular tissue combined with a conjunctival autograft at least 8 × 10 mm in size in a large number of patients with recurrent pterygium. We analyzed their postoperative clinical outcomes, recurrence rates, and complications. A conjunctival graft larger than 8 × 10 mm was chosen because removal of a large amount of fibrovascular tissue in combination with Tenon's capsule tissue is necessary to minimize the risk of recurrence after surgery [63839]. A large graft is required to cover t he resulting conjunctival defect.
In this study, the postoperative recurrence rate for pterygium was 4.0%, which was better than that of previously published reports. Kria et al. [38] and Kria et al. [39] have evaluated growth factor and angiogenesis modulation of pterygial fibroblasts in vitro and explained why extensive removal of conjunctival fibrovascular tissue and Tenon's capsule layer can reduce the rate of recurrence. Ti et al. [10] have suggested that the effectiveness of pterygium surgery is subject to a learning curve. Surgeons who had performed a larger number of pterygium excisions in combination with a conjunctival autograft were found to have a lower rate of recurrence. It was also suggested that the size of the graft and the suturing technique used might be important in preventing recurrence. In this study, all surgeries were performed by one expert surgeon who had completed more than 500 pterygium excisions in combination with conjunctival autografts, which might explain the excellent results obtained in the present study.
There were some complications in this study that occurred as a result of the large graft size. These included graft edema, donor site scarring, pyogenic granuloma, and development of conjunctival inclusion cysts.
There were also two cases of graft edema that lasted for more than three months after surgery. In both cases, the edema resolved spontaneously with time. Kim et al. [19] have also reported graft edema following conjunctival autograft with pterygium excision. In their study, graft edema persisted longer in cases that had received injections of subconjunctival bevacizumab prior to surgery. It was suggested that bevacizumab inhibited the proliferation of vessels and lymphatics and therefore delayed normal wound healing.
In this study, five patients experienced donor site scarring. The use of a thin conjunctival flap without any subconjunctival tissue or Tenon's capsule tissue, as well as minimal surgical trauma, reduces the risk of donor site scarring. Thirteen patients developed a pyogenic granuloma at the donor site. The granuloma was treated with potent steroid eye drops and resolved with time.
There were seven cases that developed inclusion cysts. These types of cysts have been reported to arise from dislocated epithelium below the surface of the conjunctiva or cornea secondary to trauma [40] or surgery [41]. Acquired conjunctival inclusion cysts are known to develop following surgical implantation of conjunctival epithelium [4142]. This complication can be prevented by paying careful attention during creation and placement of the conjunctival autograft.
The use of a nti-VEGF d rugs as a n adjuvant treatment following pterygium excision is controversial [2021222324252627]. A subconjunctival injection of bevacizumab is reportedly effective in preventing the recurrence of pterygiums without causing any significant adverse effects [2024]. Stival et al. [20] reported that a single injection of subconjunctival bevacizumab in cases with recurrent pterygium was well tolerated and decreased irritation and vascularization over the short term. Nava-Castaneda et al. [28] found that vascularization and corneal opacity were reduced in all cases of recurrent pterygium following three injections of subconjunctival bevacizumab. Therefore, the authors of that study recommended three injections of 2.5 mg/mL subconjunctival bevacizumab for the treatment of recurrent pterygium [28]. According to Fallah et al. [27], the short-term use of topical bevacizumab is a safe and effective means for delaying the recurrence of pterygiums. In contrast, some studies have concluded that bevacizumab only partially decreases conjunctival vascularization, and its effects are transient. Therefore, bevacizumab had no statistically significant effect on preventing pterygium recurrence [2125]. In this study, subconjunctival bevacizumab injections were administered at a dosage of 5.0 mg (0.2 mL) in the five cases with recurrence. After an average of 2.4 subconjunctival bevacizumab injections (range, 1 to 4), there was no progression of fibrovascular proliferation in any of the cases. Vascular endothelial growth factor has been shown to be elevated in cases with pterygium and is suggested to be involved in its pathogenesis [43444546]. Decreased anti-angiogenic factors along with increased stimulators h ave been h ypothesized t o play a r ole in t he formation and progression of pterygium [44]. The finding of abundant expression of VEGF in the pterygium supports the use of anti-VEGF therapy with the goal of reducing the number of blood vessels present and the size of the pterygium. However, tumor necrosis factor alpha, basic fibroblast growth factor, transforming growth factor beta, and platelet-derived growth factor have also been shown to correlate with the formation and recurrence of pterygium. Therefore, the use of anti-VEGF drugs alone might have a limited effect in preventing pterygium recurrence. Although it might have a limited effect on reducing the recurrence of pterygium after surgery, subconjunctival anti-VEGF injections in the early stages of pter ygium recurrence after surgery might suppress neovascularization and prevent or retard its progression.
This study had some limitations. First, because there was no control group, the effect of a large conjunctival autograft on the management of recurrent pterygium could not be compared with other treatments. Nevertheless, the recurrence rate after surgical excision of recurrent pterygiums ranges from 14.8% to 33.0% [91017]. The recurrence rate of 4.0% in this study was fairly low, and the treatment method was effective. Secondly, the procedure used to harvest the superior conjunctiva might complicate any future glaucoma filtering surgeries. Although we excluded patients who were known or suspected to have glaucoma, the average age of the patients in this study was only 56.5 ± 10.2 years. Future development of glaucoma remains a possibility, and its management could be difficult as a result of this procedure. Thirdly, there was a limited number of patients in the subconjunctival bevacizumab injection group. Therefore, this study could not verify the effects of bevacizumab on the prevention of pterygium recurrence after surgery.
In summary, we found that extensive removal of conjunctival fibrovascular tissue combined with a large conjunctival autograft appears to be a safe and effective method for the treatment of recurrent pterygium. Subconjunctival bevacizumab injections can prevent the progression of pterygium recurrence after surgery. However, the lack of randomized clinical trials on large conjunctival autograft is another limitation of this study. Therefore, prospective randomized clinical trials are necessary to compare large conjunctival autografts with other treatments and to verify the effects of subconjunctival bevacizumab injections on prevention of pterygium recurrence.
Figures and Tables
Fig. 1
Surgical conjunctival autograft procedures in pterygium surgery. (A) Preoperative recurrent nasal pterygium. (B) The pterygium head was excised, the conjunctiva allowed to retract, and the subconjunctival fibrovascular tissue excised en bloc. (C) The superior conjunctival autograft was flipped over the cornea and the limbal attachment, and then the head of the palisades of Vogt was cleaned with a blade. (D) The conjunctival autograft was placed over the cornea. The graft was rotated toward the defect site by spreading the conjunctiva while being mindful of orienting the limbal stem cell population toward the limbus. (E) Fibrin glue was applied over the bare sclera and conjunctival autograft. A drop of fibrinogen solution was placed on the bare sclera, and a drop of thrombin solution was applied to the conjunctival autograft. (F) The conjunctival autograft was spread over the bare sclera, and the edges were bonded to the surrounding health conjunctiva using McPherson forceps. (G) The donor conjunctival tissue was sutured to the recipient site using several simple interrupted sutures with 10-0 nylon (Ethilon; Johnson & Johnson Medical, Cincinnati, OH, USA) at the tissue margins. (H) After the conjunctival autograft was secured, temporary amniotic membrane transplantation was performed.

Fig. 2
Change in conjunctival injection after pterygium surgery. (A) Hypervascularized recurrent pterygium in a left eye. (B) Decreased conjunctival injection after pterygium excision with a conjunctival autograft.
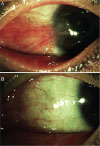
Fig. 3
A change in fibrous tissue contraction after pterygium surgery. (A) Thick fibrovascular tissue on the cornea induced ocular motility limitations. (B) Resolution of the thick fibrovascular tissue and ocular motility limitation after pterygium excision with a conjunctival autograft.
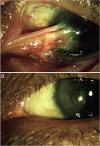
Fig. 4
Complications after pterygium surgery. (A) Graft edema. (B) Donor site scarring. (C) Pyogenic granuloma. (D) Conjunctival epithelial inclusion cyst.
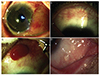
Fig. 5
A 45-year-old male experienced pterygium recurrence after pterygium surgery and received two subconjunctival bevacizumab injections. (A) Recurrent pterygium before the surgery. (B) Pterygium recurrence eight weeks postoperatively. (C) No progression of fibrovascular proliferation after two subconjunctival bevacizumab injections for six months.

References
1. Yoon KC, Mun GH, Kim SD, et al. Prevalence of eye diseases in South Korea: data from the Korea National Health and Nutrition Examination Survey 2008-2009. Korean J Ophthalmol. 2011; 25:421–433.
2. Wong WW. A hypothesis on the pathogenesis of pterygiums. Ann Ophthalmol. 1978; 10:303–308.
3. Coroneo MT. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993; 77:734–739.
4. Zauberman H. Pterygium and its recurrence. Am J Ophthalmol. 1967; 63:1780–1786.
5. Youngson RM. Recurrence of pterygium after excision. Br J Ophthalmol. 1972; 56:120–125.
6. Hirst LW. Prospective study of primary pterygium surgery using pterygium extended removal followed by extended conjunctival transplantation. Ophthalmology. 2008; 115:1663–1672.
7. Kucukerdonmez C, Akova YA, Altinors DD. Comparison of conjunctival autograft with amniotic membrane transplantation for pterygium surgery: surgical and cosmetic outcome. Cornea. 2007; 26:407–413.
8. Tananuvat N, Martin T. The results of amniotic membrane transplantation for primary pterygium compared with conjunctival autograft. Cornea. 2004; 23:458–463.
9. Shimazaki J, Kosaka K, Shimmura S, Tsubota K. Amniotic membrane transplantation with conjunctival autograft for recurrent pterygium. Ophthalmology. 2003; 110:119–124.
10. Ti SE, Chee SP, Dear KB, Tan DT. Analysis of variation in success rates in conjunctival autografting for primary and recurrent pterygium. Br J Ophthalmol. 2000; 84:385–389.
11. Kaufman SC, Jacobs DS, Lee WB, et al. Options and adjuvants in surgery for pterygium: a report by the American Academy of Ophthalmology. Ophthalmology. 2013; 120:201–208.
12. Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997; 115:1235–1240.
13. Ha SW, Park JH, Shin IH, Kim HK. Clinical analysis of risk factors contributing to recurrence of pterygium after excision and graft surgery. Int J Ophthalmol. 2015; 8:522–527.
14. Koranyi G, Artzen D, Seregard S, Kopp ED. Intraoperative mitomycin C versus autologous conjunctival autograft in surgery of primary pterygium with four-year follow-up. Acta Ophthalmol. 2012; 90:266–270.
15. Ozer A, Yildirim N, Erol N, Yurdakul S. Long-term results of bare sclera, limbal-conjunctival autograft and amniotic membrane graft techniques in primary pterygium excisions. Ophthalmologica. 2009; 223:269–273.
16. Luanratanakorn P, Ratanapakorn T, Suwan-Apichon O, Chuck RS. Randomised controlled study of conjunctival autograft versus amniotic membrane graft in pterygium excision. Br J Ophthalmol. 2006; 90:1476–1480.
17. Fallah MR, Golabdar MR, Amozadeh J, et al. Transplantation of conjunctival limbal autograft and amniotic membrane vs mitomycin C and amniotic membrane in treatment of recurrent pterygium. Eye (Lond). 2008; 22:420–424.
18. Ma DH, See LC, Hwang YS, Wang SF. Comparison of amniotic membrane graft alone or combined with intraoperative mitomycin C to prevent recurrence after excision of recurrent pterygia. Cornea. 2005; 24:141–150.
19. Kim YI, Lee GY, Kim EJ, et al. The effect of subconjunctival bevacizumab injection before conjunctival autograft for pterygium. J Korean Ophthalmol Soc. 2015; 56:847–855.
20. Stival LR, Lago AM, Figueiredo MN, et al. Efficacy and safety of subconjunctival bevacizumab for recurrent pterygium. Arq Bras Oftalmol. 2014; 77:4–7.
21. Hu Q, Qiao Y, Nie X, et al. Bevacizumab in the treatment of pterygium: a meta-analysis. Cornea. 2014; 33:154–160.
22. Ozgurhan EB, Agca A, Kara N, et al. Topical application of bevacizumab as an adjunct to recurrent pterygium surgery. Cornea. 2013; 32:835–838.
23. Hurmeric V, Vaddavalli P, Galor A, et al. Single and multiple injections of subconjunctival ranibizumab for early, recurrent pterygium. Clin Ophthalmol. 2013; 7:467–473.
24. Alhammami H, Farhood Q, Shuber H. Subconjunctival bevacizumab injection in treatment of recurrent pterygium. J Clin Exp Ophthalmol. 2013; 01. 23. DOI: 10.4172/2155-9570.1000267.
25. Lekhanont K, Patarakittam T, Thongphiew P, et al. Randomized controlled trial of subconjunctival bevacizumab injection in impending recurrent pterygium: a pilot study. Cornea. 2012; 31:155–161.
26. Mandalos A, Tsakpinis D, Karayannopoulou G, et al. The effect of subconjunctival ranibizumab on primary pterygium: a pilot study. Cornea. 2010; 29:1373–1379.
27. Fallah MR, Khosravi K, Hashemian MN, et al. Efficacy of topical bevacizumab for inhibiting growth of impending recurrent pterygium. Curr Eye Res. 2010; 35:17–22.
28. Nava-Castaneda A, Ulloa-Orozco I, Garnica-Hayashi L, et al. Triple subconjunctival bevacizumab injection for early corneal recurrent pterygium: one-year follow-up. J Ocul Pharmacol Ther. 2015; 31:106–113.
29. Safianik B, Ben-Zion I, Garzozi HJ. Serious corneoscleral complications after pterygium excision with mitomycin C. Br J Ophthalmol. 2002; 86:357–358.
30. Dougherty PJ, Hardten DR, Lindstrom RL. Corneoscleral melt after pterygium surgery using a single intraoperative application of mitomycin-C. Cornea. 1996; 15:537–540.
31. Bahar I, Kaiserman I, Lange AP, et al. The effect of mitomycin C on corneal endothelium in pterygium surgery. Am J Ophthalmol. 2009; 147:447–452.
32. Kheirkhah A, Izadi A, Kiarudi MY, et al. Effects of mitomycin C on corneal endothelial cell counts in pterygium surgery: role of application location. Am J Ophthalmol. 2011; 151:488–493.
33. Avisar R, Apel I, Avisar I, Weinberger D. Endothelial cell loss during pterygium surgery: importance of timing of mitomycin C application. Cornea. 2009; 28:879–881.
34. Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997; 104:974–985.
35. Solomon A, Pires RT, Tseng SC. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology. 2001; 108:449–460.
36. Kim HH, Mun HJ, Park YJ, et al. Conjunctivolimbal autograft using a fibrin adhesive in pterygium surgery. Korean J Ophthalmol. 2008; 22:147–154.
37. Fernandes M, Sangwan VS, Bansal AK, et al. Outcome of pterygium surgery: analysis over 14 years. Eye (Lond). 2005; 19:1182–1190.
38. Kria L, Ohira A, Amemiya T. TNP-470 (a fungus-derived inhibitor of angiogenesis) reduces proliferation of cultured fibroblasts isolated from primary pterygia: a possible drug therapy for pterygia. Curr Eye Res. 1998; 17:986–993.
39. Kria L, Ohira A, Amemiya T. Growth factors in cultured pterygium fibroblasts: immunohistochemical and ELISA analysis. Graefes Arch Clin Exp Ophthalmol. 1998; 236:702–708.
40. Barishak RY, Baruh E, Lazar M. Episcleral traumatic conjunctival inclusion cyst. Br J Ophthalmol. 1977; 61:299–301.
41. Song JJ, Finger PT, Kurli M, et al. Giant secondary conjunctival inclusion cysts: a late complication of strabismus surgery. Ophthalmology. 2006; 113:1049.
42. Williams BJ, Durcan FJ, Mamalis N, Veiga J. Conjunctival epithelial inclusion cyst. Arch Ophthalmol. 1997; 115:816–817.
43. Gebhardt M, Mentlein R, Schaudig U, et al. Differential expression of vascular endothelial growth factor implies the limbal origin of pterygia. Ophthalmology. 2005; 112:1023–1030.
44. Jin J, Guan M, Sima J, et al. Decreased pigment epithelium-derived factor and increased vascular endothelial growth factor levels in pterygia. Cornea. 2003; 22:473–477.
45. Marcovich AL, Morad Y, Sandbank J, et al. Angiogenesis in pterygium: morphometric and immunohistochemical study. Curr Eye Res. 2002; 25:17–22.
46. Lee DH, Cho HJ, Kim JT, et al. Expression of vascular endothelial growth factor and inducible nitric oxide synthase in pterygia. Cornea. 2001; 20:738–742.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download