Abstract
Purpose
To evaluate and compare the toxic effects of eyedrops containing a fixed combination of 2.0% dorzolamide and 0.5% maleate timolol with or without preservatives on rabbit corneal endothelium.
Methods
This study was performed with 22 eyes of New Zealand white rabbits. Dorzolamide/timolol eyedrops with preservative (Cosopt group) or without preservative (Cosopt-S group) were diluted with a balanced salt solution at a 1 : 1 ratio. We injected 0.1 mL of diluted Cosopt into the anterior chamber of left eyes and an equal volume of diluted Cosopt-S into the anterior chamber of right eyes. Corneal thickness, corneal haze, and conjunctival injection were measured before and 24 hours after treatment. Endothelial damage was compared between both eyes by vital staining (alizarin red/trypan blue staining), live/dead cell assay, TUNEL assay, and scanning electron microscopy.
Results
Corneal endothelial damage was severe in the Cosopt group. Cosopt-treated eyes exhibited remarkable corneal edema and prominent apoptosis of endothelial cells. In addition, the live/dead cell assay revealed many dead cells in the endothelium, and scanning electron microscopy analysis showed that corneal endothelial cells exhibited a partial loss of microvilli on the surface as well as extensive destruction of intercellular junctions. However, in the Cosopt-S group, corneal edema was mild and the damage to the corneal endothelium was minimal.
Preservatives in ophthalmic solutions can prevent contamination and extend the shelf life of products. However, such preservatives can also induce cytotoxic responses as well as allergic reactions [12345]. The adverse effects associated with these preservatives seem to be more significant for glaucoma patients, mainly because of their chronic use of one or more preservative-containing agents [6]. Therefore, considerable efforts have been made in the recent years by pharmaceutical companies to develop less toxic glaucoma medications such as preservative-free preparations [7].
Although the effects of the various active ingredients in glaucoma medications on the corneal endothelium have not been fully elucidated, topical dorzolamide, a carbonic anhydrase inhibitor, is known to have potential effects [89]. Specifically, dorzolamide may attenuate bicarbonate efflux, leading to corneal edema. Irreversible corneal decompensation was reported in nine glaucoma patients with endothelial compromise after topical dorzolamide treatment [10]. Indeed, in clinical practice, anti-glaucoma eyedrops containing dorzolamide are not recommended to patients with compromised corneal endothelium. However, it is unclear whether topical dorzolamide can cause corneal edema in eyes with normal corneal endothelium in vivo.
Many surgeons now perform sutureless cataract surgery. In the early postoperative period, tears on the ocular surface can enter the anterior chamber through unstable wounds [111213], and even more so if the epithelial barrier is disrupted. In such cases, drugs or preservatives can have harmful effects on the corneal endothelium. Thus, the present study was designed to determine whether administration of eyedrops containing dorzolamide to the corneal endothelium can induce corneal edema. In addition, we compared the toxic effect of dorzolamide based on the presence or absence of preservatives.
Eleven New Zealand white rabbits (22 eyes) weighing between 2.0 and 2.5 kg were used in this study. The left eye of each rabbit was treated with a fixed combination of 2.0% dorzolamide and 0.5% maleate timolol with 0.0075% benzalkonium chloride added as a preservative (Cosopt; Merck & Co., Whitehouse Station, NJ, USA), while the right eye of each rabbit was treated with a unit-dose preservative-free formulation of the dorzolamide/timolol combination (Cosopt-S, Merck & Co.). The use of the rabbits conformed to the Association for Research in Vision and Ophthalmology's Statement for the Use of Animals in Ophthalmic and Vision Research.
Two different anti-glaucoma eyedrops were diluted with balanced salt solution to a ratio of 1 : 1, and then 0.1 mL of either eyedrop was injected into the anterior chambers of 11 rabbits with the aid of a surgical microscope under general anesthesia induced by intramuscular injection of zolazepam and tiletamine (Zoletil; Virbac, Carros, France). Using aseptic technique, we inserted a 30-gauge needle through the anterior conjunctiva and sclera into the anterior chamber, and subsequently removed about 0.1 mL of aqueous material from the anterior chamber. The syringe was then removed, and the syringe containing 0.1 mL of the study eyedrops was attached and injected.
Central corneal thickness was measured with an ultra-sound corneal pachymeter (BV International, Clermont-Ferrand, France) before and 24 hours after injection. Corneal haze was evaluated according to Fantes' classification [14]. Specifically, a corneal haze grade of 0 is assigned for a totally clear cornea; grade 0.5, trace haze faintly detectable with broad illumination; grade 1, minimal haze easily seen with broad illumination; grade 2, mild haze easily visible with direct focal slit illumination; grade 3, moderate opacity obscuring iris details; and grade 4, severe opacity blocking the ability to observe the anterior chamber structure. Conjunctival and limbal vascular injection was also graded from 0 (none) to 4 (very severe).
All rabbits were euthanized in a carbon dioxide chamber 24 hours after intracameral injections, and the eyes were then enucleated to facilitate further studies (e.g., dual vital staining, live/dead cell assay, TUNEL assay, and scanning electron microscopy [SEM]). In order to evaluate corneal endothelial integrity, dual staining of corneal endothelium with trypan blue and alizarin red was performed in three eyes of each group. Isolated corneas were placed endothelial side up in a Teflon corneal cup and a 7.5 mm corneal button was cut from the center using a surgical corneal punch. Trypan blue was added dropwise to cover the endothelium of the corneal disc and the stain was poured off after 2 minutes. The corneal disc was then briefly rinsed twice in normal saline, drained to remove excess saline, and finally placed back in the corneal cup. The endothelial layer was then covered with alizarin red (0.4%, pH 4.2) for 3 minutes and again rinsed twice in saline after pouring away the staining reagent. After the staining procedure, corneal discs were fixed in a glutaraldehyde solution for 15 minutes. The corneal disc was then mounted endothelial side up on a microscope slide with a central cavity to accommodate the thickness of the corneal button and permit examination under a light microscope (BX51TF; Olympus, Tokyo, Japan).
Endothelial cell viability was evaluated using a live/dead viability/cytotoxicity kit (Molecular Probes, Eugene, OR, USA) in three eyes from each group. Staining was performed according to the manufacturer's instructions. Live cells, which were distinguished by the presence of ubiquitous intracellular esterase activity, appeared green, whereas dead cells with damaged membranes stained red. The number of dead cells was calculated in 5 consecutive microscopic fields from each eye under high magnification (×200) by a blinded observer.
Another three eyes of each group were immediately frozen in optimum cutting temperature compound (Tissue-Tek; Miles Laboratories, Elkahart, IN, USA) with liquid nitrogen, and then central corneal sections (10 µm thick) were cut using a cryostat at -20℃ and placed on slides coated with polylysine. Some specimens were stained with hematoxylin and eosin for histopathological observation, while the remaining unused specimens were subjected to TUNEL staining for analysis of endothelial cell apoptosis. The TUNEL assay was performed using the ApopTag Red In Situ Apoptosis Detection Kit (cat no. S7165; Chemicon International, Temecula, CA, USA). Photomicrographs were also taken by fluorescence confocal microscope and the number of apoptotic endothelial cells was counted from 5 consecutive microscopic fields under high magnification (×400) by a blinded observer. DAPI (4',6-diamidino-2-phenylindole) was used to counterstain nuclei.
SEM was performed on the last two eyes of each group. For SEM analysis, corneas were prefixed in 2% glutaraldehyde in 0.1 M phosphate buffer and post-fixed for 2 hours in 1% osmic acid dissolved in phosphate-buffered saline. The specimens were treated in a graded series of ethanol and t-butyl alcohol and dried in a freeze dryer (ES-2030; Hitachi, Tokyo, Japan). Next, the specimens were coated with platinum using an ion coater (Eiko IB-5; Eiko Engineering, Ibaragi, Japan) and finally observed with an FE-SEM (S-4700, Hitachi).
IBM SPSS ver. 20 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. A nonparametric Mann-Whitney U-test was used to compare variables between the two groups. A p-value of <0.05 was considered statistically significant.
Before injection, central corneal thickness of two groups showed no statistically significant differences (Cosopt group, 359.9 µm; Cosopt-S group, 358.7 µm). After injection, the increase of central corneal thickness was significantly greater in the Cosopt group than in the Cosopt-S group (p < 0.001) (Table 1). The degree of corneal haze, limbal, and conjunctival vascular injection were also greater in the Cosopt group (p < 0.001) (Table 1, Fig. 1A and 1B).
Hematoxylin and eosin staining revealed a very edematous cornea in the Cosopt group compared to the Cosopt-S group. Further, many endothelial cells were lost in the Cosopt group but not in the Cosopt-S group (Fig. 1C and 1D).
All corneas from the Cosopt group exhibited extensive areas of endothelial cell damage resulting in nuclei stained with trypan blue (Fig. 2A). Endothelial cells in the Cosopt group were enlarged and had lost their normal hexagonal morphology. In contrast, corneas from the Cosopt-S group maintained a regular hexagonal-shaped endothelial layer (Fig. 2B), although some large endothelial cells were observed.
The live/dead cell assay performed 24 hours after injection revealed that many endothelial cells in the Cosopt group were dead as evidenced by red-stained nuclei (Fig. 2C). However, in the Cosopt-S group, dead cells were rarely observed (Fig. 2D). The median number of dead cells from 5 consecutive microscopic fields (×400) on each eye was 28 in the Cosopt group and 2 in the Cosopt-S group (p < 0.001) (Fig. 3A).
TUNEL staining demonstrated that distinct apoptosis of endothelial cells was present only in the Cosopt group, and not the Cosopt-S group (Fig. 3B and 3C). The median number of TUNEL-positive endothelial cells, which were counted from 5 consecutive microscopic fields (×400), was 4 in the Cosopt group and 0 in the Cosopt-S group (p < 0.001) (Fig. 3A).
Under scanning electron microscopy, the corneal endothelium in the Cosopt group partially lost microvilli on the cell surface and the intercellular junctions were extensively destroyed (Fig. 4A). However, the corneal endothelium in the Cosopt-S group showed a uniform hexagonal appearance with regular cell borders and distinct microvilli on the cell surface (Fig. 4B).
Ness et al. [15] recently reported that the Durasite bioadhesive delivery system in topical antibiotics can block the trabecular meshwork and have a toxic effect on rabbit corneal endothelial cells when introduced intracamerally. Immediately after cataract surgery or penetrating keratoplasty, topical eyedrops can penetrate into the anterior chamber through unstable wounds. Sutureless clear corneal surgery also can result in the tear film moving in and out of the eye during blinking if the wound leaks [11], which can be exacerbated if the corneal epithelium is injured [16]. Under such conditions, the concentration of eyedrops in the anterior chamber would be higher than in eyes with an intact epithelium.
Fixed combination anti-glaucoma eyedrops have recently been introduced for better patient compliance and greater intraocular pressure reduction. Cosopt is a fixed combination of 2.0% dorzolamide and 0.5% maleate timolol. Fixed combination eyedrops also have the advantage of decreased ocular toxicity due to the need for reduced dosing of anti-glaucoma eyedrops containing preservatives. However, the cytotoxicity of preservatives has been a considerable problem for long-term use in patients with glaucoma or dry eyes [17]. Therefore, preservative-free artificial tears are gaining popularity among patients with dry eyes, and preservative-free unit dose anti-glaucoma eyedrops have recently been introduced. Along these lines, Cosopt-S is a unit-dose preservative-free formulation of the dorzolamide/timolol combination.
Although the potential effects of dorzolamide on corneal endothelial cells have not been fully elucidated, topical carbonic anhydrase inhibitors may attenuate the bicarbonate efflux by blocking carbonic anhydrase in the corneal endothelium, leading to corneal edema [8]. However, previous studies on the effect of dorzolamide on corneal deswelling showed that dorzolamide does not slow the recovery from induced corneal edema in normal subjects as well as in patients with glaucoma or ocular hypertension [1819]. Irreversible corneal decompensation was reported in nine patients after topical treatment with dorzolamide [10]. Specifically, all nine cases reported in that study underwent intraocular surgery and exhibited compromised corneal endothelia. Although it is unclear how topical treatment of dorzolamide affects corneal endothelium and induces corneal decompensation, the authors suggested that dorzolamide can cause irreversible corneal edema in glaucoma patients with compromised corneal endothelia [10]. On the other hand, the topical dorzolamide eyedrops used in their study of corneal decompensation contained 0.005% benzalkonium chloride as a preservative. Thus, we hypothesized that the damage to the corneal endothelium may have been due to the preservative rather than dorzolamide, or possibly that the preservative may have caused additional damage to corneal endothelia in conjunction with dorzolamide.
In this study, we compared the toxic effects on the corneal endothelium using two different eyedrops containing the same anti-glaucoma components, but one with preservative and the other without. In order to directly examine the toxic effects of the two formulations of eyedrops, we injected one formulation each into the respective anterior chambers and evaluated toxic damage to the corneal endothelium after 24 hours. In the eyes injected with the preservative-containing Cosopt, corneal endothelial damage was severe. Likewise, Cosopt-treated eyes exhibited severe corneal edema and prominent apoptosis of endothelial cells, as well as numerous dead cells as determined by a live/dead cell assay. SEM analysis showed that corneal endothelial cells also exhibited a partial loss of microvilli on the surface as well as extensive destruction of intercellular junctions. However, corneal edema was mild in the eyes injected with the preservative-free formulation Cosopt-S, and damage to the corneal endothelium was minimal. Based on the results of this study, we concluded that the main cause of endothelial toxicity was the preservative, and not the active ingredients of the anti-glaucomatous agents. Therefore, anti-glaucoma eyedrops containing preservative may have the potential to cause damage to the corneal endothelium as well as to the ocular surface, especially during the early postoperative period or in cases of an epithelial defect. In these conditions, it seem be safer to use preservative-free anti-glaucoma eyedrops, as several studies have already demonstrated that there is no difference in intraocular pressure reduction between preservative-containing and preservative-free formulations [202122232425]. Moreover, benzalkonium chloride expression has been detected in the trabecular meshwork, corneal endothelium, lens, and retina after topical drop installation, which may contribute to toxicity in these tissues [26].
Our study had a few limitations. In order to evaluate toxicity, we injected the anti-glaucoma eyedrops directly into the anterior chamber. Although the eyedrops were diluted, the concentration of anti-glaucoma components and preservative was most likely greater than the concentrations that could be obtained following topical administration. However, it is difficult to predict the true amount of eyedrops that enters through clear corneal wounds during the early postoperative period. While it is unusual in clinical practice that a high concentration of eyedrops could directly enter the anterior chamber directly, we reasoned that direct injection of dorzolamide eyedrops into the anterior chamber could represent a case whereby the corneal endothelium is affected, leading to corneal edema. Thus, long-term use of topical anti-glaucoma eyedrops may affect the function of the corneal endothelium and eventually can cause corneal edema. Therefore, our study design may provide information on the maximal toxic effects of anti-glaucoma eyedrops on the corneal endothelium in the presence or absence of preservative. Another limitation of this study was the use of rabbits, whose corneal endothelial cells are different from human corneal endothelial cells in terms of cell proliferation. Indeed, rabbit corneal endothelial cells can proliferate and rabbit corneas can recover their normal clarity after endothelial injury on a time scale of several days to weeks [2728]. To overcome this limitation, we evaluated the toxic effects of anti-glaucoma eyedrops only 24 hours after injection. Therefore, we could demonstrate distinct differences between endothelial damage caused by preservative-containing and preservative-free eyedrops.
In conclusion, we demonstrated that the main cause of endothelial toxicity upon treatment with a dorzolamide-containing solution was due to the preservative, and not the active ingredient of this anti-glaucoma medication. Thus, it may be safer to use preservative-free anti-glaucoma eyedrops during the early postoperative period or in cases where enhanced corneal penetration is a concern.
Figures and Tables
Fig. 1
(A) Slit lamp photograph of an eye 24 hours after injection of Cosopt. A rabbit eye in the Cosopt group showing severe corneal haze and conjunctival vascular injection. (B) Slit lamp photograph of an eye 24 hours after injection of Cosopt-S. A rabbit eye in the Cosopt-S group showing minimal corneal haze and conjunctival vascular injection, the extent of which was much more mild than that of Cosopt-treated eyes. (C) Histopathologic photomicrograph of a rabbit cornea 24 hours after injection of Cosopt. Cornea showing severe stromal edema. Many endothelial cells were lost (inset). (D) Histopathologic photomicrograph of a rabbit cornea 24 hours after injection of Cosopt-S. Significant stromal edema is absent. A single layer of endothelium is well observed (inset) (hematoxylin and eosin, ×40; inset ×400).
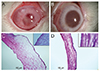
Fig. 2
(A) Vital staining of corneal endothelium with trypan blue and alizarin red 24 hours after Cosopt injection. Extensive endothelial cell damage is noted, resulting in nuclei stained with trypan blue. The corneal endothelial cells are enlarged and have lost their normal hexagonal pattern (×400). (B) Vital staining of corneal endothelium with trypan blue and alizarin red 24 hours after injection of Cosopt-S. The corneal endothelial cells exhibit a normal hexagonal pattern, and some enlarged endothelial cells can be observed (×400). (C) Live/dead cell assay on corneal endothelium 24 hours after injection of Cosopt. Many endothelial cells are dead as evidenced by red-stained nuclei (×200). (D) Live/dead cell assay on corneal endothelium 24 hours after injection of Cosopt-S. Few dead cells are present (×200).
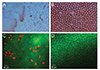
Fig. 3
(A) Comparison of the number of dead cells from live/dead cell assay and TUNEL(+) cells in 5 consecutive microscopic fields between the Cosopt and Cosopt-S groups (×400). (B) TUNEL stain of rabbit cornea 24 hours after Cosopt injection. Several TUNEL(+) cells are present in the endothelial cell layer. (C) TUNEL stain of rabbit cornea 24 hours after Cosopt-S injection. TUNEL-positive cells are absent (×400).
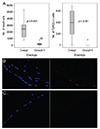
Fig. 4
(A) Photograph of scanning electron microscopy 24 hours after Cosopt injection. Corneal endothelial cells have lost microvilli on the cell surface and intercellular junctions are extensively destroyed (×500). (B) Photograph of scanning electron microscopy 24 hours after Cosopt-S injection. Corneal endothelial cells continue to exhibit a hexagonal appearance with distinct microvilli on the cell surface (×500). SE = secondary electron; U = upper detector.

Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2042054). This study was supported in part by Alumni of Department of Ophthalmology, Korea University College of Medicine in 2014.
References
1. Lapalus P, Ettaiche M, Fredj-Reygrobellet D, et al. Cytotoxicity studies in ophthalmology. Lens Eye Toxic Res. 1990; 7:231–242.
2. Guzman-Aranguez A, Calvo P, Ropero I, Pintor J. In vitro effects of preserved and unpreserved anti-allergic drugs on human corneal epithelial cells. J Ocul Pharmacol Ther. 2014; 30:790–798.
3. Mantelli F, Tranchina L, Lambiase A, Bonini S. Ocular surface damage by ophthalmic compounds. Curr Opin Allergy Clin Immunol. 2011; 11:464–470.
4. Baudouin C, Labbe A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010; 29:312–334.
5. Ayaki M, Noda Y, Yaguchi S, et al. Cytotoxicity of antiglaucoma ophthalmic solutions for human corneal endothelial cells. Nihon Ganka Gakkai Zasshi. 2009; 113:576–582.
6. Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001; 108:1943–1953.
7. Inoue K, Iwasa M, Wakakura M, Tomita G. Effects of BAK-free travoprost treatment for 3 years in patients with normal tension glaucoma. Clin Ophthalmol. 2012; 6:1315–1319.
8. Srinivas SP, Ong A, Zhai CB, Bonanno JA. Inhibition of carbonic anhydrase activity in cultured bovine corneal endothelial cells by dorzolamide. Invest Ophthalmol Vis Sci. 2002; 43:3273–3278.
9. Wirtitsch MG, Findl O, Heinzl H, Drexler W. Effect of dorzolamide hydrochloride on central corneal thickness in humans with cornea guttata. Arch Ophthalmol. 2007; 125:1345–1350.
10. Konowal A, Morrison JC, Brown SV, et al. Irreversible corneal decompensation in patients treated with topical dorzolamide. Am J Ophthalmol. 1999; 127:403–406.
11. Behrens A, Stark WJ, Pratzer KA, McDonnell PJ. Dynamics of small-incision clear cornea wounds after phacoemulsification surgery using optical coherence tomography in the early postoperative period. J Refract Surg. 2008; 24:46–49.
12. Herretes S, Stark WJ, Pirouzmanesh A, et al. Inflow of ocular surface f luid into the anterior chamber after phacoemulsification through sutureless corneal cataract wounds. Am J Ophthalmol. 2005; 140:737–740.
13. Taban M, Sarayba MA, Ignacio TS, et al. Ingress of India ink into the anterior chamber through sutureless clear corneal cataract wounds. Arch Ophthalmol. 2005; 123:643–648.
14. Fantes FE, Hanna KD, Waring GO 3rd, et al. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990; 108:665–675.
15. Ness PJ, Mamalis N, Werner L, et al. An anterior chamber toxicity study evaluating Besivance, AzaSite, and Ciprofloxacin. Am J Ophthalmol. 2010; 150:498–504.e1.
16. Liu GS, Trope GE, Basu PK. Ultrastructural effects of topical betoptic, betagan, and timoptic on the rabbit corneal endothelium. J Ocul Pharmacol. 1989; 5:329–342.
17. Ayaki M, Yaguchi S, Iwasawa A, Koide R. Cytotoxicity of ophthalmic solutions with and without preservatives to human corneal endothelial cells, epithelial cells and conjunctival epithelial cells. Clin Experiment Ophthalmol. 2008; 36:553–559.
18. Egan CA, Hodge DO, McLaren JW, Bourne WM. Effect of dorzolamide on corneal endothelial function in normal human eyes. Invest Ophthalmol Vis Sci. 1998; 39:23–29.
19. Giasson CJ, Nguyen TQ, Boisjoly HM, et al. Dorzolamide and corneal recovery from edema in patients with glaucoma or ocular hypertension. Am J Ophthalmol. 2000; 129:144–150.
20. Baudouin C, de Lunardo C. Short-term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br J Ophthalmol. 1998; 82:39–42.
21. Lewis RA, Katz GJ, Weiss MJ, et al. Travoprost 0.004% with and without benzalkonium chloride: a comparison of safety and efficacy. J Glaucoma. 2007; 16:98–10.
22. Konstas AG, Quaranta L, Katsanos A, et al. Twenty-four hour efficacy with preservative free tafluprost compared with latanoprost in patients with primary open angle glaucoma or ocular hypertension. Br J Ophthalmol. 2013; 97:1510–1515.
23. Rouland JF, Traverso CE, Stalmans I, et al. Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br J Ophthalmol. 2013; 97:196–200.
24. Daull P, Buggage R, Lambert G, et al. A comparative study of a preservative-free latanoprost cationic emulsion (Catioprost) and a BAK-preserved latanoprost solution in animal models. J Ocul Pharmacol Ther. 2012; 28:515–523.
25. Renieri G, Fuhrer K, Scheithe K, et al. Efficacy and tolerability of preservative-free eye drops containing a fixed combination of dorzolamide and timolol in glaucoma patients. J Ocul Pharmacol Ther. 2010; 26:597–603.
26. Rasmussen CA, Kaufman PL, Kiland JA. Benzalkonium chloride and glaucoma. J Ocul Pharmacol Ther. 2014; 30:163–169.
27. Maurice D, Perlman M. Permanent destruction of the corneal endothelium in rabbits. Invest Ophthalmol Vis Sci. 1977; 16:646–649.
28. Valdez-Garcia JE, Lozano-Ramirez JF, Zavala J. Adult white New Zealand rabbit as suitable model for corneal endothelial engineering. BMC Res Notes. 2015; 8:28.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download